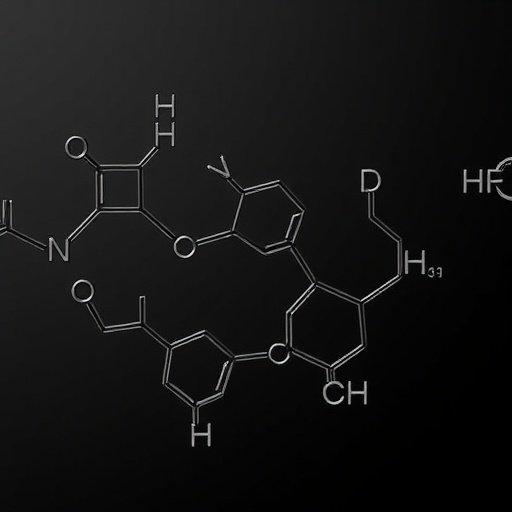
In a landmark advancement poised to redefine conjugated diene chemistry, a collaborative research team led by professors Liang’an Chen of Nanjing Normal University and Liangliang Song of Nanjing Forestry University has unveiled a novel palladium-catalyzed methodology that achieves unprecedented control over regio-, chemo-, and stereoselective coupling reactions. This breakthrough paves the way for the streamlined synthesis of multi-substituted conjugated dienes, compounds that are pivotal intermediates and building blocks in pharmaceuticals, natural product synthesis, and advanced materials science.
The research capitalizes on a neutral oxidative addition-reductive elimination reaction mechanism, a cornerstone in transition metal catalysis, which allows for precise manipulation of molecular architecture. By employing an innovative ligand angle control strategy, the team successfully steered the reaction pathway to favor selective nucleophilic attack on propargyl alcohol esters, a challenging substrate class known for its versatile reactivity but difficult regiochemical control. This strategic ligand design fine-tunes the electronic and steric environment around the palladium center, enabling selective formation of C–C or C–X bonds, where X denotes a range of nucleophiles.
The scope of nucleophiles employed in this study is impressively broad and includes fluorides, phenols, alcohols, carboxylic acids, and amides. This versatility highlights the robustness and generality of the catalytic system, as it tolerates diverse functional groups without sacrificing selectivity. The generated multi-substituted conjugated dienes feature precise stereochemical disposition, a crucial attribute for downstream transformations, particularly those involving pericyclic processes or selective functionalizations with bioactive targets.
At the mechanistic core, the palladium catalyst facilitates oxidative addition of the propargyl ester to form a palladium(II) intermediate, followed by regioselective nucleophilic attack directed via the ligand’s spatial configuration. Subsequent reductive elimination releases the functionalized diene while regenerating the active palladium(0) species, thus ensuring catalytic turnover. This approach avoids traditional challenges, such as side reactions or overfunctionalization, frequently encountered in conjugated diene synthesis.
The practical application of this chemistry is underscored by the ease with which these functionalized 1,3-dienes engage in downstream reaction manifolds. The team demonstrated their utility in cycloaddition reactions, including Diels-Alder processes that construct complex polycyclic frameworks with high diastereo- and enantioselectivity. Furthermore, these dienes act as privileged intermediates in coupling reactions, which expand their molecular diversity and enable late-stage functionalization — an invaluable tactic in drug discovery and natural product derivatization.
To validate the method’s real-world potential, Chen and Song’s team applied their protocol to modify natural products and bioactive molecules, sufficiently showcasing the methodology’s amenability to structurally complex and sensitive substrates. This application exemplifies a significant stride toward practical synthesis within medicinal chemistry, where modifications to scaffolds are often limited by harsh or non-selective reaction conditions.
A particularly striking feature of this work is its open accessibility, as it has been published as an open access research article in CCS Chemistry, the flagship journal of the Chinese Chemical Society. This open publication model facilitates rapid dissemination and incorporation of these innovative catalytic principles into the global scientific community’s toolkit, potentially accelerating advances in synthetic strategy design.
This breakthrough underscores the growing importance of ligand engineering in transition metal catalysis, showcasing how subtle alterations to catalyst geometry profoundly influence reaction outcomes. With the precise modulation of the ligand environment, scientists gain an unprecedented level of control over chemoselectivity and stereoselectivity, which has long been a holy grail in organic synthesis, especially for constructing polyunsaturated frameworks.
The implications of this research extend beyond academic curiosity, as conjugated dienes are central to the production of polymers, agrochemicals, and pharmaceuticals. The ability to synthesize intricately substituted dienes with high selectivity and functional group compatibility offers a powerful platform for tailoring the physicochemical properties of the resulting molecules, ultimately impacting materials science and drug development.
Moreover, the team’s approach addresses a longstanding synthetic challenge concerning the selective functionalization of propargyl substrates, which ordinarily undergo side reactions such as rearrangements or polymerization under catalytic conditions. By harnessing oxidative addition-reductive elimination cycles with a ligand that dictates the spatial preference of the palladium center, the method suppresses undesired pathways, setting a new precedent for selectivity in palladium-catalyzed transformations.
In sum, this innovative study by the Nanjing research team is a testament to the synergy between sophisticated catalyst design and practical synthetic application. By unlocking new reactivity and selectivity patterns, their method enriches the synthetic chemist’s repertoire and holds promise for accelerating the synthesis of functional materials and complex natural products with precision.
As this research gains traction in the chemical community, one can anticipate a surge in catalytic strategies inspired by the ligand angle control philosophy, promoting further exploration of oxidative addition-reductive elimination mechanisms across other transition metals and substrate classes. This paradigm not only fosters creativity in reaction design but also promotes molecular complexity and sustainability in chemical synthesis.
The unveiling of this palladium-catalyzed approach heralds an exciting era for conjugated diene chemistry where selectivity, efficiency, and functional diversity converge, charting new territories for synthetic innovation and discovery.
Subject of Research: Palladium-Catalyzed Regio-, Chemo-, and Stereoselective Coupling of Propargyl Alcohol Esters for Multi-Substituted Conjugated Dienes
News Publication Date: Not specified
Web References: Not specified
References: Published as an open access research article in CCS Chemistry, the flagship journal of the Chinese Chemical Society.
Image Credits: EurekAlert! Media Service