In recent years, mounting evidence has pointed to the profound impact environmental chemicals can have not only on individuals directly exposed to them but also on subsequent generations. A striking new study published in Nature Communications brings this issue into sharper focus by elucidating how prenatal exposure to propylparaben—a widely used preservative found in countless personal care products—can induce transgenerational reproductive dysfunction in mice. This groundbreaking work not only advances our understanding of how everyday chemical exposure can compromise female fertility but also raises urgent questions about the long-term consequences of seemingly harmless consumer compounds.
At the heart of this investigation is the concept of diminished ovarian reserve (DOR), a condition characterized by a reduced number of viable eggs and consequently, a lower likelihood of conception. It is well known that ovarian reserve naturally declines with age, but environmental insults can exacerbate this deterioration, leading to premature ovarian aging. Li et al. employed a multifaceted approach combining molecular biology, reproductive physiology, and advanced epigenetic analyses to demonstrate that prenatal exposure to propylparaben triggers ovarian reserve depletion in female offspring, effects that persist not only in the directly exposed generation but surprisingly extend to at least two subsequent generations.
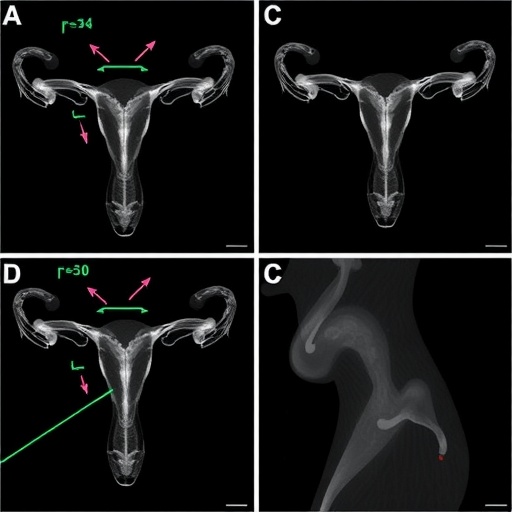
The researchers began by administering controlled doses of propylparaben to pregnant mice during a critical window of fetal gonadal development. Subsequent examination of the adult female offspring revealed a significant reduction in the population of primordial follicles—the pool of immature eggs that sustain fertility over a female’s reproductive lifespan. Quantitative assessments of follicle counts established that these deficits were dose-dependent, with higher prenatal exposure correlating with more pronounced ovarian reserve diminishment. Importantly, this effect was not limited to the initially exposed progeny; researchers observed comparable follicle depletion in the F2 and F3 generations despite the absence of direct chemical exposure, underscoring a heritable epigenetic mechanism at play.
Delving deeper into the molecular underpinnings, the study highlighted altered patterns of DNA methylation and histone modifications within the ovarian tissue of affected animals. These epigenetic changes are believed to disrupt the regulation of key genes involved in follicle development, survival, and apoptosis. Particularly notable was the dysregulation of genes implicated in the PI3K-AKT signaling pathway, a critical regulator of follicular activation and growth. The misexpression of these genes likely accelerates the premature activation and subsequent depletion of primordial follicles, effectively exhausting the ovarian reserve earlier than normal.
Moreover, transcriptomic profiling of ovarian tissue revealed a concerted downregulation of genes responsible for antioxidative responses, suggesting increased susceptibility to oxidative stress—a known contributor to ovarian aging and follicular attrition. This multifaceted molecular assault helps explain the observed phenotypic outcome of diminished follicle counts and impaired ovarian function. Functional fertility assays corroborated these molecular findings, with affected females displaying reduced litter sizes and prolonged intervals to conception compared to controls.
Perhaps the most unsettling discovery was the transgenerational transmission of these deleterious effects. The study’s design included breeding exposed females with control males and assessing reproductive parameters in successive offspring not directly exposed to propylparaben. The persistence of ovarian reserve reduction and altered epigenetic landscapes in these later generations suggested that the chemical exposure induced heritable epimutations in the germline. This phenomenon aligns with the growing body of work on epigenetic inheritance, challenging traditional genetic paradigms by showing that environmental factors can imprint lasting marks beyond direct DNA sequence alterations.
The potential translational implications of this study are profound. Propylparaben is omnipresent in cosmetics, shampoos, lotions, and even some food packaging due to its antimicrobial properties. Human epidemiological data have hinted at links between parabens and endocrine disruption, but elucidating a clear causal relationship has been challenging. This mouse model provides a crucial biological framework suggesting that prenatal exposure to such compounds could compromise female fertility across generations. Given the rising incidence of infertility and concerns about early ovarian aging worldwide, reevaluating exposure limits and regulatory policies surrounding parabens becomes imperative.
From a public health perspective, this study underscores the need for heightened vigilance regarding prenatal environmental exposures. The fetal period is exquisitely sensitive to endocrine disruptors, and the ramifications of perturbations during this time may only materialize much later—sometimes in offspring never directly exposed to the toxic insult. The demonstration that propylparaben exposure in utero can precipitate epigenetic modifications with lasting reproductive consequences demands further research into other common chemicals with similar mechanisms.
Interestingly, the study also paves the way for future inquiries exploring therapeutic interventions. If the epigenetic alterations induced by propylparaben exposure can be identified and characterized in detail, targeted epigenetic editing or pharmacological treatments may be developed to restore normal gene expression patterns and preserve ovarian reserve. While such strategies are speculative at this stage, this research lays the groundwork for innovative approaches to combat environmentally induced infertility.
Furthermore, the research team employed cutting-edge single-cell sequencing technologies that offered unprecedented resolution of ovarian cell populations affected by paraben exposure. These analyses revealed shifts in the cellular composition and altered signaling networks within the ovarian microenvironment, which may contribute to follicular attrition. The integration of such advanced omics techniques with classical reproductive biology exemplifies the innovative multidisciplinary approach that is essential for tackling complex environmental health issues.
The authors also emphasized the importance of studying male reproductive parameters in future investigations, as parabens have been implicated in male reproductive toxicity as well. Comprehensive assessments encompassing both sexes and multiple generations will better define the full scope of reproductive risks posed by parabens. Meanwhile, public awareness campaigns should be intensified to educate consumers about potential reproductive hazards associated with widely used preservatives.
Overall, this landmark study from Li and colleagues signals a paradigm shift in how we understand the long-term effects of chemical exposure on reproductive health. The identification of transgenerational ovarian reserve depletion induced by a common preservative not only advances the field of environmental reproductive toxicology but also ignites urgent debate regarding chemicals once considered safe. By revealing the silent legacy of prenatal propylparaben exposure, this work calls for immediate reevaluation of regulatory frameworks and renewed commitment to protecting future generations from invisible but devastating reproductive insults.
As infertility rates unexpectedly climb and the age of first pregnancy continues to rise globally, insights gleaned from such animal models illuminate potential environmental contributors to this trend. The intersection of epigenetics, developmental toxicology, and reproductive medicine promises to unravel the complex etiologies underlying reproductive decline, catalyzing new preventive and therapeutic strategies. Until then, caution is warranted with widespread exposure to endocrine-disrupting chemicals, and pregnant individuals should be particularly vigilant.
In conclusion, the meticulous dissection by Li et al. of the repercussions of prenatal propylparaben exposure illustrates the vulnerability of the developing female reproductive system to environmental chemicals. The heritable epigenetic modifications identified provide a mechanistic explanation for how chemical insults can transcend generations, inflicting durable reproductive dysfunction. This seminal work challenges us to reconsider the safety profiles of everyday compounds and sparks vital conversations bridging science, policy, and public health. Ultimately, such research paves the way for a healthier future where reproductive longevity is safeguarded from the unseen threats embedded in our modern environment.
Subject of Research:
Transgenerational effects of prenatal propylparaben exposure on ovarian reserve and fertility in mice.
Article Title:
Transgenerational inheritance of diminished ovarian reserve triggered by prenatal propylparaben exposure in mice.
Article References:
Li, M., Wu, Y., Wei, S. et al. Transgenerational inheritance of diminished ovarian reserve triggered by prenatal propylparaben exposure in mice. Nat Commun 16, 8289 (2025). https://doi.org/10.1038/s41467-025-63440-z
Image Credits: AI Generated