A research team led by Prof. YE Shandong and Prof. ZHENG Mao from the First Affiliated Hospital of the University of Science and Technology of China (USTC), in collaboration with researchers from the Anhui Medical University, identified a novel mechanism by which the body regulates oxidative stress pressure, offering new insights into how cells respond to oxidative stress. The study was published in Nature Communications.

Credit: USTC
A research team led by Prof. YE Shandong and Prof. ZHENG Mao from the First Affiliated Hospital of the University of Science and Technology of China (USTC), in collaboration with researchers from the Anhui Medical University, identified a novel mechanism by which the body regulates oxidative stress pressure, offering new insights into how cells respond to oxidative stress. The study was published in Nature Communications.
Aging and weight gain both cause stress for the body, mainly manifested as excessive production of reactive oxygen species (ROS). But excess ROS can lead to metabolic diseases associated with obesity and aging, such as diabetes and fatty liver. Although antioxidants and ROS scavengers can alleviate metabolic dysfunction, long-term antioxidant treatment has potential safety issues, and there are no ideal drugs available at present. Therefore, it is crucial to understand the molecular mechanisms underlying lipid metabolism dysfunction caused by oxidative stress.
Metabolic-associated fatty liver disease is a pathological condition characterized primarily by the accumulation of triglycerides within hepatocytes, manifesting mainly as lipid droplet accumulation. Lipid droplets (LDs), as the main organelle for lipid storage, normally play a role in regulating energy metabolism.
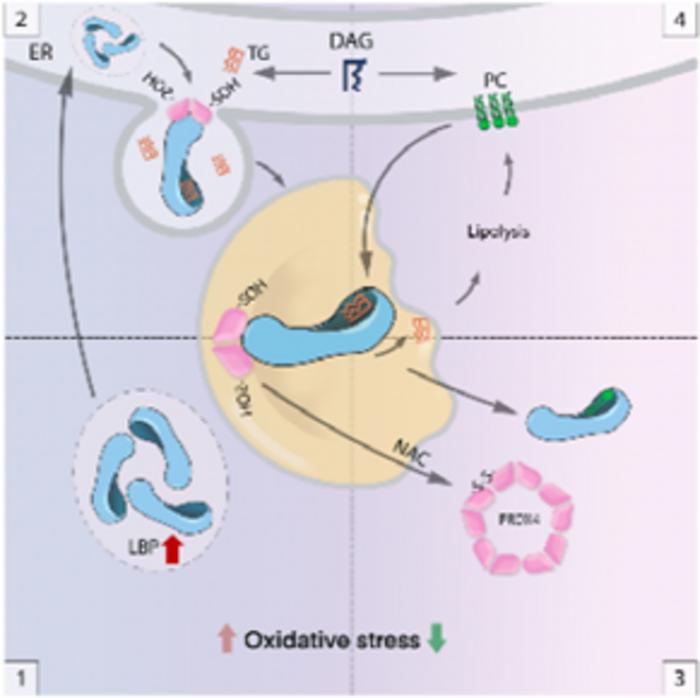
Under oxidative stress, LDs accumulate a large number of unsaturated fatty acids triglyceride (UFA-TG) susceptible to ROS attack to prevent further peroxidation and thus maintain lipid homeostasis. However, the molecular mechanism underlying “oxidative stress avoidance” under oxidative stress is poorly understood and the cellular sorting mechanism of UFA-TG urgently needs to be elucidated.
The study indicated that the expression level of lipopolysaccharide-binding protein (LBP) increased and aggregated in lipid droplets when cells were under oxidative stress. LBP had a lipid-capturing activity, capturing lipids through its hydrophobic structure at the C terminus and transporting them to lipid droplets, thereby controlling lipid-oxidative homeostasis.
It was also found that treatment with the reductant N-acetyl-L-cysteine could scavenge intracellular ROS and increase phospholipid synthesis. Phospholipids could competitively bind LBP with triglycerides, promote LBP translocation out of lipid droplets and promote lipolysis. However, in an environment where oxidative stress was not eliminated, the use of phospholipids to treat fatty liver may cause more serious hepatocyte damage.
Peroxiredoxin 4, as a sensor of cellular redox signaling, regulated the LBP/triglyceride lipid droplet shuttling process by interacting with LBP to maintain cellular redox homeostasis. In addition, long-term stress stimulation upregulated LBP expression, which led to insulin resistance and obesity.
This work provides new insights into the development of novel therapeutic strategies based on redox homeostasis regulation for alleviating oxidative stress-induced metabolic dysfunction and offers a new direction for the prevention and treatment of metabolic diseases.
Journal
Nature Communications
Article Title
Lipopolysaccharide binding protein resists hepatic oxidative stress by regulating lipid droplet homeostasis
Article Publication Date
13-Apr-2024