In a groundbreaking study published in Cell Death Discovery, researchers have unveiled a promising therapeutic approach for neuroblastoma, a devastating pediatric cancer that originates from neural crest cells. The investigation spearheaded by Shokraie, Lechermeier, Bordihn, and colleagues presents compelling evidence that cyclin-dependent kinase (CDK) inhibitors not only promote differentiation in neuroblastoma cells but also considerably enhance their sensitivity to retinoic acid. This dual mechanism opens up a novel avenue to improve existing treatment regimens for this aggressive malignancy, potentially transforming patient outcomes.
Neuroblastoma represents one of the most common solid tumors in infancy and early childhood, often characterized by its ability to evade differentiation signals and adopt a highly malignant, proliferative state. Conventional therapies, including chemotherapy, surgery, and radiation, have limited efficacy, particularly in high-risk cases. Retinoic acid (RA), a derivative of vitamin A, has been used as a differentiation-inducing agent, aiming to redirect malignant neuroblastoma cells toward a more mature, less aggressive phenotype. However, resistance to RA is a significant obstacle, curbing its therapeutic utility in many patients.
The study in question centers on the critical role of CDKs, a family of serine/threonine kinases that orchestrate cell cycle progression and influence cellular differentiation. Overactivity of specific CDK isoforms has been linked to uncontrolled proliferation in various cancers. By targeting CDKs with small-molecule inhibitors, the researchers aimed to disrupt this pathological cell cycle regulation, inducing a differentiation program within neuroblastoma cells. The team’s innovative approach rested on the premise that CDK inhibition might normalize aberrant cell cycle signals, thereby restoring the cells’ intrinsic ability to mature upon RA exposure.
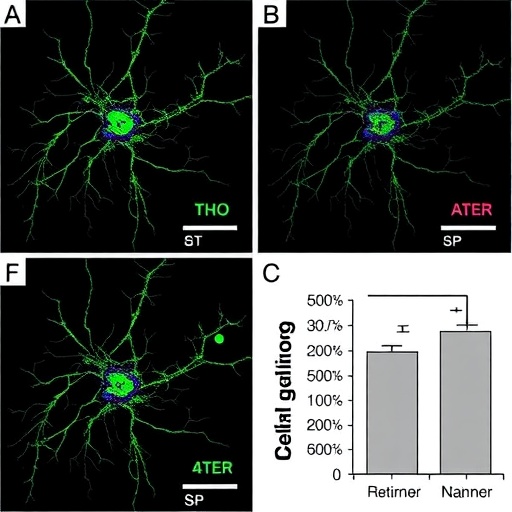
Employing a suite of in vitro experiments, the authors first demonstrated that treatment with selective CDK inhibitors led to marked morphological changes in neuroblastoma cell lines, indicative of differentiation. Cells exhibited neurite outgrowth and altered expression of differentiation-associated markers, signaling a shift away from the undifferentiated, proliferative phenotype. Quantitative analyses further confirmed these phenotypic changes, reinforcing the hypothesis that CDK activity plays a pivotal role in maintaining the malignant state.
Beyond morphological evidence, the molecular fingerprint of gene expression changes under CDK inhibition was carefully dissected. Transcriptomic profiling revealed upregulation of neuronal differentiation genes, accompanied by downregulation of proliferation-associated transcripts. This transcriptional reprogramming highlights the multifaceted impact of CDK inhibitors and suggests that they orchestrate complex cascades to tip the balance from cell division toward maturation. The findings illuminate previously underappreciated connections between cell cycle regulators and differentiation pathways in neuroblastoma.
Crucially, the study explored the synergistic potential of combining CDK inhibitors with retinoic acid treatment. While RA monotherapy induces differentiation in susceptible cells, the addition of CDK inhibitors significantly amplified this effect, sensitizing resistant cell populations to RA’s differentiating influence. This combinatorial strategy was tested across multiple neuroblastoma cell lines, illustrating broad applicability and robustness of the therapeutic benefit. Enhanced induction of differentiation markers and greater reduction in cell viability underscored the synergistic interaction.
Mechanistically, the combination appeared to converge on shared signaling networks, including modulation of retinoic acid receptor activity and downstream effectors. CDK inhibitors seem to prime the chromatin landscape and transcriptional machinery, heightening cellular responsiveness to RA. This facilitates a more profound reprogramming of gene expression, effectively overcoming barriers that limit RA efficacy when used alone. These insights provide a mechanistic rationale to propel clinical investigation of combination therapies.
Furthermore, the research delineates how inhibition of specific CDK isoforms impacts neuroblastoma pathology. The data emphasize the nuanced roles of individual CDKs beyond canonical cell cycle progression. By dissecting these roles, the study paves the way for precision-medicine approaches where tailored inhibitors targeting distinct CDKs could be selected based on tumor genotype and phenotype. Such customization holds promise for maximizing therapeutic impact while minimizing adverse effects.
In addition to detailed cellular and molecular analyses, the authors also evaluated functional consequences of the therapeutic interventions. Differentiated neuroblastoma cells displayed decreased clonogenic potential and diminished capacity for anchorage-independent growth, hallmark features of tumorigenicity. These findings underscore that the induced differentiation correlates with loss of malignant characteristics, crucial for translating laboratory observations into effective clinical strategies.
The implications of this study extend beyond neuroblastoma, suggesting that CDK inhibitors might prove beneficial in other cancers where differentiation blockade contributes to malignancy. The idea of combining cell cycle modulators with differentiation agents represents an elegant and rational therapeutic paradigm. Importantly, the existing clinical use of RA and CDK inhibitors facilitates potential rapid translation into clinical trials, accelerating the timeline from bench to bedside.
Despite the promise, the authors caution that further investigations are requisite to address outstanding questions. These include the long-term stability of induced differentiation, potential resistance mechanisms to combined therapy, and optimal dosing regimens to maximize efficacy while curtailing toxicity. Animal model studies and eventual clinical trials will be instrumental in validating the efficacy and safety observed in cellular models.
The study also touches on the broader biological significance of CDKs in developmental contexts and cancer. By revealing how CDK activity intersects with differentiation pathways in neuroblastoma, the work underscores fundamental principles of cell biology and oncogenic transformation. Such knowledge deepens our understanding of tumor biology and identifies vulnerabilities amenable to therapeutic exploitation.
In conclusion, the research by Shokraie and colleagues marks a pivotal advance in neuroblastoma treatment strategies. Through meticulous dissection of molecular mechanisms and therapeutic synergy, the study provides a robust foundation for developing combination treatments that harness the power of CDK inhibitors and retinoic acid. This dual attack on cell proliferation and differentiation blockade offers renewed hope for improving outcomes in pediatric neuroblastoma patients facing limited therapeutic options.
As this research progresses toward clinical application, it exemplifies the critical importance of integrating molecular insights with translational goals. Harnessing the interplay between cell cycle regulation and differentiation not only expands the therapeutic arsenal but also exemplifies innovation in combating childhood cancer. The dynamic nature of neuroblastoma biology and the urgent need for more effective therapies make this combined CDK inhibitor and RA strategy a beacon for future oncology research and treatment.
Subject of Research: Neuroblastoma, CDK inhibition, cellular differentiation, retinoic acid sensitivity, pediatric oncology
Article Title: CDK inhibitors promote neuroblastoma cell differentiation and increase sensitivity to retinoic acid—a promising combination strategy for therapeutic intervention
Article References:
Shokraie, F., Lechermeier, L., Bordihn, P. et al. CDK inhibitors promote neuroblastoma cell differentiation and increase sensitivity to retinoic acid—a promising combination strategy for therapeutic intervention. Cell Death Discov. 11, 363 (2025). https://doi.org/10.1038/s41420-025-02637-z
Image Credits: AI Generated