Antibiotic medicines became a popular treatment for bacterial infections in the early 20th century and emerged as a transformational tool in human health. Through the middle of the century novel antibiotics were regularly developed in the medication’s golden age.

Credit: Pogliano Labs, UC San Diego
Antibiotic medicines became a popular treatment for bacterial infections in the early 20th century and emerged as a transformational tool in human health. Through the middle of the century novel antibiotics were regularly developed in the medication’s golden age.
But then bacteria evolved. They found new ways to evade antibiotic treatments, rendering many useless. As new antibiotic sources dried up, bacterial infections escalated into today’s global health crisis of antibiotic resistance.
Scientists now look to an unusual ally, viruses, to help counter this rising threat. Recently, researchers have focused on viruses known as bacteriophages as a new tool to treat and disarm antibiotic-resistant bacteria. Special attention has been placed on “jumbo” phages — viruses recently discovered to feature extremely large genomes — that could be tapped as special delivery agents that can not only kill bacteria but could be engineered to deliver antibiotics directly to the source of infection.
But in order to deliver novel therapeutics through phage, scientists must first understand the extraordinary biological makeup and mechanisms inside these mysterious viruses.
University of California San Diego School of Biological Sciences researchers and their colleagues at UC Berkeley’s Innovative Genomics Institute and the Chulalongkorn University in Bangkok have taken a substantial step forward in deciphering several key functions within jumbo phages.
“These jumbo phages have large genomes that in theory could be manipulated to carry payloads that more effectively kill bacteria,” said Joe Pogliano, a UC San Diego professor in the School of Biological Sciences and senior author of the new paper, which was published April 30 in the Proceedings of the National Academy of Sciences. “The problem is that their genome is enclosed so it’s not easy to access. But now we’ve discovered some of its key elements.”
As described in the paper, research led by School of Biological Sciences graduate student Chase Morgan focused on jumbo Chimalliviridae phages that were found to replicate inside bacteria by forming a compartment that resembles the nucleus inside the cells of humans and other living organisms. The Chimalliviridae’s nucleus-like compartment separates and selectively imports certain proteins that allow it to replicate inside the host bacteria. But how this process unfolds had been a puzzling part of the process.
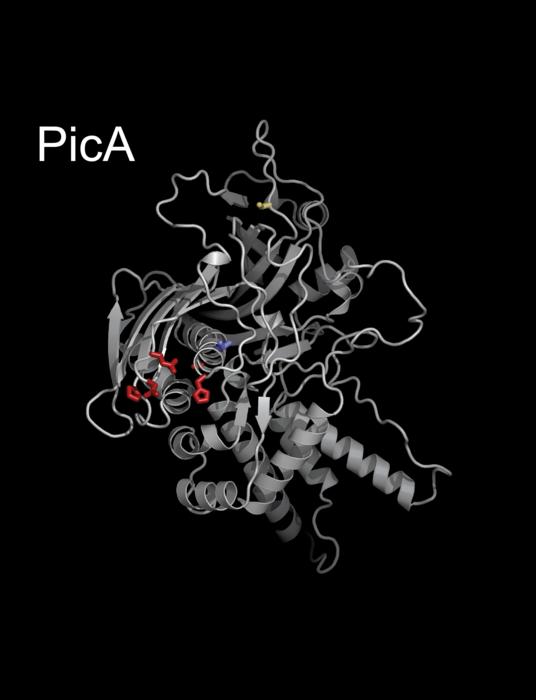
Using new genetic and cell biology tools, Morgan and his colleagues identified a key protein, which they named “protein importer of Chimalliviruses A,” or PicA, that acts as a type of nightclub bouncer, selectively trafficking proteins by granting entry inside the nucleus for some but denying access for others. PicA, they found, coordinates cargo protein trafficking across the protective shell of the phage nucleus.
“Just the fact that this virus is able to set up this incredibly complex structure and transport system is really amazing and the likes of which we haven’t seen before,” said Morgan. “What we think of as complex biology is usually reserved for higher life forms with humans and our tens of thousands of genes, but here we are seeing functionally analogous processes in a comparatively tiny viral genome of only approximately 300 genes. It’s probably the simplest selective transport system that we know of.”
Using CRISPRi-ART, a programmable RNA tool for studying genomes, the researchers were able to demonstrate that PicA is an essential component of the Chimalliviridae nucleus development and replication process.
“Without the simplicity and versatility of RNA-targeting CRISPR technologies, directly asking and answering these questions would be nearly impossible. We are really excited to see how these tools unravel the mysteries encoded by phage genomes,” said co-author Ben Adler, a postdoctoral scholar working under Nobel Prize-winning CRISPR pioneer Jennifer Doudna.
Bacteria and viruses have engaged in a type of arms race for billions of years, each evolving to counter the other’s adaptations. The researchers say the sophisticated PicA transportation system is a result of that intense, ongoing evolutionary competition. The system has evolved to be both highly flexible and highly selective, allowing only key beneficial elements inside the nucleus. Without the PicA system, the bacteria’s defensive proteins would work their way inside and sabotage the virus’ replication process.
Such information is vital as scientists with the Howard Hughes Medical Institute (HHMI)-funded Emerging Pathogens Initiative and UC San Diego’s Center for Innovative Phage Applications and Therapeutics strive to lay the groundwork to eventually genetically program phage to treat a variety of deadly diseases.
“We really didn’t have any understanding of how the protein import system worked or which proteins were involved previously, so this research is the first step in understanding a key process that’s critical for these phage to successfully replicate,” said School of Biological Sciences graduate student Emily Armbruster, a paper coauthor. “The more we understand these essential systems, the better we will be able to engineer phage for therapeutic use.
Future targets for such genetically programmed viruses include Pseudomonas aeruginosa bacteria, which are known to cause potentially fatal infections and pose risks for patients in hospitals. Other promising targets include E. coli and Klebsiella which can cause chronic and recurrent infections and, in some cases, enter the blood stream which can be life threatening.
Journal
Proceedings of the National Academy of Sciences
Method of Research
Experimental study
Subject of Research
Cells
Article Title
An essential and highly selective protein import pathway encoded by nucleus-forming phage
Article Publication Date
30-Apr-2024
COI Statement
Professors Kit and Joe Pogliano have an equity interest in Linnaeus Bioscience Inc. and receive income. Professor Doudna is a cofounder of Caribou Biosciences, Editas Medicine, Scribe Therapeutics, Intellia Therapeutics and Mammoth Biosciences. Doudna also is a scientific advisory board member of Vertex, Caribou Biosciences, Intellia Therapeutics, Scribe Therapeutics, Mammoth Biosciences, Algen Biotechnologies, Felix Biosciences, The Column Group and Inari. She is chief science advisor to Sixth Street, a Director at Johnson & Johnson, Altos and Tempus and has research projects sponsored by Apple Tree Partners and Roche. The Regents of the University of California have patents issued and pending for CRISPR technologies on which Doudna, Cress, Adler, Joe and Kit Pogliano and Armbruster are inventors.