Microglia are the brain’s immune cells known to play a vital role in maintaining neural function and responding to potential threats. However, when the brain is subjected to hyperosmotic stress—a condition characterized by elevated extracellular sodium levels, the microglial response can become exaggerated, leading to potentially harmful effects. Understanding the mechanisms behind this heightened response is crucial for the treatment of hypernatremia-induced neurological dysfunctions.

Credit: Yoshihisa Sugimura from Fujita Health University
Microglia are the brain’s immune cells known to play a vital role in maintaining neural function and responding to potential threats. However, when the brain is subjected to hyperosmotic stress—a condition characterized by elevated extracellular sodium levels, the microglial response can become exaggerated, leading to potentially harmful effects. Understanding the mechanisms behind this heightened response is crucial for the treatment of hypernatremia-induced neurological dysfunctions.
To address this issue, a research team led by Professor Yoshihisa Sugimura from the Department of Endocrinology, Diabetes, and Metabolism at Fujita Health University, Japan explored the effects of hyperosmotic stress on microglial cells. The study was co-authored by Sachiho Fuse, Haruki Fujisawa, and Atsushi Suzuki from the same department and the findings were published in the journal Peptides on June 20, 2024.
In this study, the researchers focused on the role of NFAT5, a transcription factor known for regulating cellular responses to osmotic stress. Despite NFAT5’s well-established role in other cell types, its function in microglia was not well understood. Hence, the researchers aimed to determine whether NFAT5 influences the expression of NOS2, an enzyme involved in NO production, under hyperosmotic conditions. Additionally, the researchers evaluated the potential therapeutic effects of minocycline, an anti-inflammatory drug, on microglial responses to hyperosmotic stress.
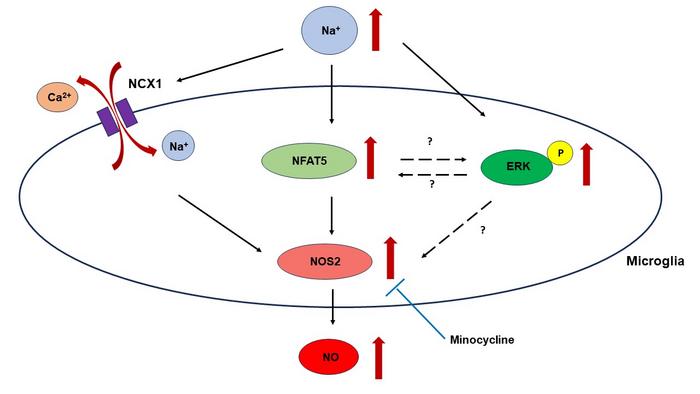
First, the researchers subjected the BV-2 microglial cells to hyperosmotic stress by increasing extracellular sodium concentrations by 20 and 40 mM above normal levels. They measured changes in NFAT5 expression, NOS2 expression, and NO production. Additionally, they explored the role of the Na+/Ca2+ exchanger, NCX in this process and investigated the effects of minocycline.
Elaborating on the findings, Prof. Sugimura says, “The results revealed that acute and chronic hyperosmotic stress significantly increased NFAT5 expression in BV-2 microglial cells. This upregulation of NFAT5 was associated with enhanced NOS2 expression and increased NO production, indicating that NFAT5 plays a crucial role in modulating microglial responses to hyperosmotic stress.” The study also highlighted the role of the NCX in this process. Adding further, Prof. Sugimura says, “Hyperosmotic stress triggered Ca2+ efflux through NCX, contributing to elevated NOS2 expression and NO production.” This finding aligns with previous research on macrophages revealing unique aspects of microglial physiology. Interestingly, this process seemed to operate independently of NFAT5, suggesting multiple activation pathways are involved in the microglial response to hyperosmotic stress.
Additionally, the researchers found that minocycline effectively inhibited NOS2 expression and NO production induced by hyperosmotic stress, suggesting its potential as a therapeutic strategy for managing conditions associated with elevated sodium levels. However, the inhibitory effect of minocycline appeared to be independent of NFAT5, indicating other underlying mechanisms.
Moving forward, the researchers recommend undertaking future investigations into the differences in microglial behavior under acute versus chronic hyperosmotic stress and the precise role of NFAT5 in these processes. Validating these findings with animal models will provide a deeper understanding of hyperosmotic stress’s impact on microglial function in more complex biological contexts. Additionally, investigating other potential therapeutic agents and further examining how minocycline acts on microglia could lead to the development of more targeted and effective strategies to address electrolyte imbalances and their effects on neurological health.
In conclusion, these findings shed light on the cellular mechanisms of microglial activation in response to hyperosmotic stress, highlighting the roles of NFAT5 and NCX, and supporting the therapeutic potential of minocycline. The study provides valuable insights for researchers and clinicians tackling the challenges of neurological dysfunctions linked to hypernatremia.
***
Reference
Title of original paper: Effects of hypernatremia on the microglia
Journal: Peptides
About Fujita Health University
Fujita Health University is a private university situated in Toyoake, Aichi, Japan. It was founded in 1964 and houses one of the largest teaching university hospitals in Japan in terms of the number of beds. With over 900 faculty members, the university is committed to providing various academic opportunities to students internationally. Fujita Health University has been ranked eighth among all universities and second among all private universities in Japan in the 2020 Times Higher Education (THE) World University Rankings. THE University Impact Rankings 2019 visualized university initiatives for sustainable development goals (SDGs). For the “good health and well-being” SDG, Fujita Health University was ranked second among all universities and number one among private universities in Japan. The university became the first Japanese university to host the “THE Asia Universities Summit” in June 2021. The university’s founding philosophy is “Our creativity for the people (DOKUSOU-ICHIRI),” which reflects the belief that, as with the university’s alumni and alumnae, current students also unlock their future by leveraging their creativity.
About Professor Yoshihisa Sugimura from Fujita Health University
Dr. Yoshihisa Sugimura is a Professor at the Department of Endocrinology, Diabetes, and Metabolism at Fujita Health University, Japan. His research expertise encompasses central diabetes insipidus, rabphilin-3A, arginine vasopressin, autoimmune hypophysitis, hyponatremia, neuronal function, and osmotic demyelination syndrome. With over 85 publications in esteemed journals such as the Journal of the American Society of Nephrology and The Journal of Clinical Endocrinology and Metabolism and with 1,950 citations, Prof. Sugimura is a prominent figure in his field. Additionally, he has authored more than 20 books covering a broad range of research topics.
Funding information
This work was supported by JSPS KAKENHI (Grant Numbers 20K08919 to YS, 22K16229 to HF), Salt Science Research Foundation No. 22C2, YOKOYAMA Foundation for Clinical Pharmacology, Hori Science and Arts Foundation, and Nitto Foundation.
Journal
Peptides
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Effects of hypernatremia on the microglia
Article Publication Date
20-Jun-2024
COI Statement
None