In a groundbreaking study set to reshape our understanding of Parkinson’s disease pathogenesis, researchers have unveiled that triplication of the SNCA gene profoundly disrupts cellular homeostasis and extracellular matrix integrity in human midbrain organoids, initiating pathological cascades well before overt neurodegeneration becomes evident. This pioneering work, led by Statoulla et al., delves deep into the mechanistic underpinnings linking genetic abnormalities to the earliest molecular disturbances in dopaminergic neurons, offering unprecedented insights critical for therapeutic intervention.
The SNCA gene, encoding alpha-synuclein, has long stood at the epicenter of Parkinson’s disease research. While mutations and duplications in SNCA were previously implicated in familial Parkinson’s, triplication represents a more severe genetic aberration that dramatically amplifies alpha-synuclein expression levels. The study harnessed cutting-edge bioengineering techniques to cultivate human midbrain organoids—three-dimensional mini-brain constructs derived from human pluripotent stem cells—that faithfully recapitulate the cellular diversity and architecture of the human midbrain, the region chiefly compromised in Parkinson’s pathology.
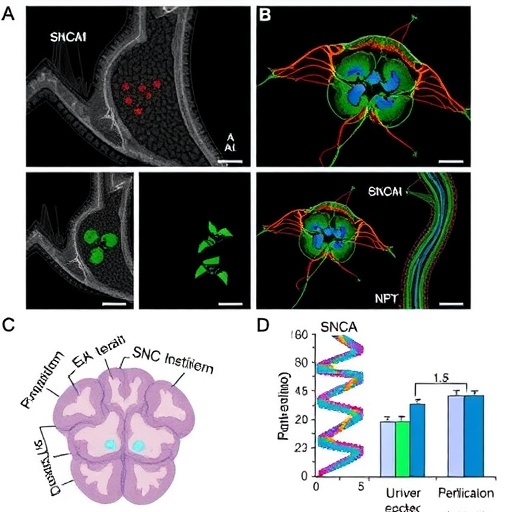
Employing these midbrain organoids with SNCA triplication, the researchers executed an array of sophisticated molecular assays, imaging modalities, and proteomic analyses to unravel how excessive alpha-synuclein perturbs intracellular protein homeostasis, or proteostasis, a delicate balance tightly regulated through protein synthesis, folding, and degradation pathways. The findings illuminate that proteostatic collapse emerges as an early event, characterized by the accumulation of misfolded alpha-synuclein species, impaired autophagic flux, and dysregulation of key chaperone proteins involved in maintaining protein quality control.
Beyond intracellular disruption, the study compellingly details alterations in the extracellular environment orchestrated by SNCA triplication. The extracellular matrix (ECM), a complex meshwork of proteins and polysaccharides providing structural and biochemical support to neuronal cells, exhibited significant remodeling. Changes in ECM composition and stiffness were observed, suggesting that aberrant alpha-synuclein expression reconfigures the extracellular neural landscape, potentially influencing neuron-glia interactions and synaptic connectivity.
One of the most striking revelations of the research lies in the temporal dissociation between early molecular disturbances and late-stage neurodegeneration. The midbrain organoids harboring SNCA triplication demonstrated clear signs of proteostatic imbalance and ECM disruption well before any appreciable neuronal death. This temporal sequence underscores a critical window for therapeutic targeting prior to irreversible neuronal loss, shifting the paradigm toward early detection and intervention strategies in Parkinson’s disease management.
Moreover, the study utilized high-resolution confocal microscopy and quantitative spatial transcriptomics to map the cellular heterogeneity and regional vulnerability within the organoids. Dopaminergic neurons exhibited pronounced susceptibility to SNCA-mediated toxicity, correlating with localized ECM alterations and intracellular stress markers, providing a spatial context to the molecular pathology. This approach not only validates the organoid platform as a superior model system but also mirrors the selective neurodegeneration observed in patient brains.
Importantly, targeting components of the proteostasis network yielded promising results in mitigating alpha-synuclein accumulation and preserving neuronal integrity within these organoids. Pharmacological modulators of autophagy and molecular chaperones were shown to partially restore proteostatic equilibrium, offering a beacon of hope for therapeutic development. Similarly, interventions aimed at normalizing ECM composition reversed some of the extracellular abnormalities, highlighting the interplay between intra- and extracellular pathogenic processes.
The intricate crosstalk between proteostasis and extracellular architecture proposed by this research pioneers a more holistic understanding of neurodegenerative pathology. It suggests that effective therapies must address not only intracellular protein aggregation but also the extracellular environment that sustains neuronal structure and connectivity, broadening the scope of future drug discovery pipelines.
By leveraging innovative stem cell technologies and multi-omic analyses, the study charts a comprehensive molecular atlas of SNCA triplication-induced pathologies. This atlas serves as a valuable resource for the scientific community seeking to decipher the complex interdependencies governing Parkinson’s disease progression, affording new biomarkers for early diagnosis and response monitoring.
This research also emphasizes the value of human organoid models over traditional animal models, particularly in capturing human-specific genomic and epigenetic contexts that modify disease phenotypes. The translational relevance is considerable, enabling the preclinical testing of candidate drugs in systems that better reflect human neurobiology and disease heterogeneity.
Furthermore, the implications extend beyond Parkinson’s disease. Since proteostasis and ECM alterations are common themes in various neurodegenerative disorders, insights gained here could inform a wider spectrum of diseases characterized by proteinopathy and extracellular dysregulation, such as Alzheimer’s disease and amyotrophic lateral sclerosis.
The meticulous delineation of the sequential and spatially-resolved impact of SNCA triplication also opens avenues for precision medicine. Stratifying patients based on genetic burden and molecular signatures gleaned from accessible biomarkers may one day tailor interventions to individual disease trajectories, maximizing efficacy while minimizing adverse effects.
In conclusion, Statoulla and colleagues’ seminal work redefines the pathogenic timeline of Parkinson’s disease by identifying early molecular derangements in proteostasis and extracellular matrix architecture as primary drivers preceding neurodegeneration. Their study not only elucidates fundamental disease mechanisms but also underscores the therapeutic promise of targeting both intracellular and extracellular pathways in the earliest stages of Parkinson’s pathology, potentially altering the course of this debilitating disorder.
Subject of Research: Parkinson’s disease pathology related to SNCA gene triplication in human midbrain organoids
Article Title: SNCA triplication disrupts proteostasis and extracellular architecture prior to neurodegeneration in human midbrain organoids
Article References:
Statoulla, E., Zafeiri, M., Chalkiadaki, K. et al. SNCA triplication disrupts proteostasis and extracellular architecture prior to neurodegeneration in human midbrain organoids. npj Parkinsons Dis. (2026). https://doi.org/10.1038/s41531-026-01292-0
Image Credits: AI Generated