In recent years, significant advancements in the understanding of esophageal cancer have shed light on the molecular mechanisms that play pivotal roles in its progression. A study by Feng, Yan, and Ge has revealed a critical role of the USP1 gene in the modulation of cancerous behavior through its regulatory effects on CDC25A and CDK1. This groundbreaking research opens new avenues for targeted therapeutic strategies in esophageal cancer, a malignancy known for its aggressive nature and poor prognosis.
Esophageal cancer, characterized by its high mortality rate, remains a major health concern globally. This malignancy primarily manifests in two forms: squamous cell carcinoma and adenocarcinoma. Understanding the cellular and molecular underpinnings of these cancers is essential for developing effective treatment options. The complexity of esophageal cancer further escalates as it often presents at advanced stages, complicating early detection and intervention.
Integral to understanding cancer biology is the ubiquitin-proteasome system (UPS), which is responsible for protein degradation and regulation within the cell. Inhibition or malfunction of this system can lead to the accumulation of oncogenic proteins, fostering tumor growth and survival. In this context, ubiquitin-specific protease 1 (USP1) has emerged as a key player. By deubiquitinating target proteins, USP1 can modulate their stability and activity, making it a molecule of interest in cancer research.
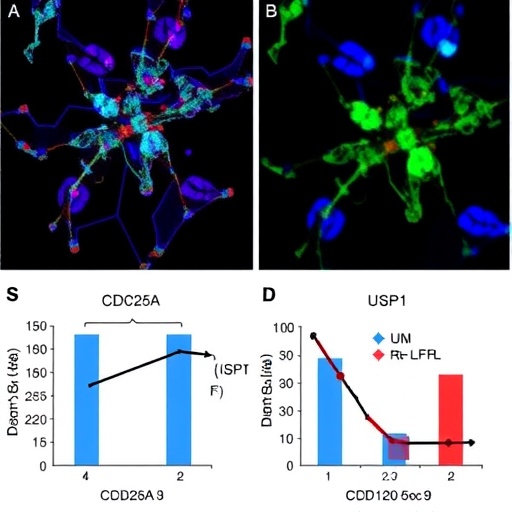
In the study, the authors focus on the role of USP1 in relation to CDC25A, a dual-specificity phosphatase that plays a crucial role in cell cycle regulation. CDC25A functions to activate cyclin-dependent kinases (CDKs), which are essential for the progression through various phases of the cell cycle. The dysregulation of CDC25A is often observed in different cancers, including esophageal cancer, suggesting its involvement in oncogenic processes.
Through experimental validation, the researchers elucidate the function of USP1 in stabilizing CDC25A by removing ubiquitin moieties from it. This is significant, as stabilized CDC25A can, in turn, enhance the expression of CDK1, a cyclin-dependent kinase that drives the transition from the G2 phase to mitosis. The interplay among USP1, CDC25A, and CDK1 creates a feedback loop that significantly influences cell proliferation and tumor growth.
The findings of this research not only enhance our understanding of esophageal cancer but also highlight potential therapeutic targets. By inhibiting USP1, it may be possible to decrease CDC25A levels, leading to reduced CDK1 activity and, consequently, hindered cancer cell proliferation. Such targeted therapeutic strategies could transform the current landscape of esophageal cancer treatment, which is often limited to surgery, chemotherapy, and radiation.
Moreover, the implications of the study extend beyond esophageal cancer. The pathways involving USP1, CDC25A, and CDK1 are conserved across various cancer types, positioning USP1 as a potential pan-cancer target. By further exploring these molecular interactions, researchers could unveil new insights into the treatment of other malignancies that share similar regulatory pathways.
The study by Feng and colleagues is a testament to the importance of molecular biology in elucidating the complexities of cancer. It serves as a reminder that understanding the intricate signaling networks within cells can pave the way for innovative treatment modalities. As the research community continues to unravel the mechanisms driving cancer progression, the promise of targeted therapies becomes ever more attainable.
The authors emphasize that further studies are needed to validate their findings in clinical settings and to evaluate the potential side effects of USP1 inhibition. It is critical to understand whether the therapeutic targeting of USP1 would affect normal cells as well, and if so, how this could be managed in treatment regimens.
As this research gains traction, the interest in identifying and developing USP1 inhibitors could escalate. Pharmaceutical companies may begin to explore compounds that can target USP1 effectively. The challenge will lie in ensuring that such inhibitors are selective, minimizing off-target effects while maximizing therapeutic efficacy.
As we look to the future, the integration of molecular insights from studies like this into clinical applications holds promise for improving patient outcomes in esophageal cancer. With the ongoing advancements in cancer therapeutics and increased understanding of tumor biology, the vision of precision medicine becomes increasingly viable.
Feng, Yan, and Ge’s research not only marks a significant milestone in cancer biology but also ignites hope for patients battling esophageal cancer. By targeting the underlying molecular mechanisms driving tumor progression, we may one day develop effective treatments that significantly improve survival rates and quality of life for those affected by this dire illness.
In conclusion, the intersection of molecular biology and cancer therapy continues to evolve, and the research conducted by Feng and colleagues serves as a crucial contribution to this dynamic field. The work underscores the necessity for ongoing investigation into cancer pathways, with the collective aim of translating these findings into clinical success.
Subject of Research: The regulatory role of USP1 in esophageal cancer, focusing on CDC25A deubiquitination and CDK1 expression.
Article Title: USP1 regulates esophageal cancer progression through CDC25A deubiquitination to regulate CDK1 expression.
Article References: Feng, J., Yan, Z. & Ge, J. USP1 regulates esophageal cancer progression through CDC25A deubiquitination to regulate CDK1 expression. 3 Biotech 16, 47 (2026). https://doi.org/10.1007/s13205-025-04663-1
Image Credits: AI Generated
DOI: https://doi.org/10.1007/s13205-025-04663-1
Keywords: USP1, esophageal cancer, CDC25A, CDK1, ubiquitin-proteasome system, targeted therapy, cancer biology, molecular mechanisms.