In the relentless pursuit of understanding cancer biology, researchers continually strive to unlock the immune response mechanisms that shape tumor immunity. A recent study titled “SOAT1 in ovarian cancer cells regulates immune response mediated by CD8+ T cells,” authored by He, J., Siu, M.K., Long, R., et al., delves into the intricate relationship between lipid metabolism and immune modulation in ovarian cancer. This work, published in the esteemed Journal of Ovarian Research, sheds light on the important role of SOAT1, a member of the sterol O-acyltransferase family, in influencing the behavior of CD8+ T lymphocytes.
Ovarian cancer has long been recognized for its aggressive nature and poor prognosis, often due to late-stage diagnosis and a complex tumor microenvironment that can evade immune detection. Understanding the underlying mechanisms that facilitate this evasion is critical for the development of more effective therapies. The research conducted by He and colleagues provides compelling evidence that SOAT1 is not merely a bystander in ovarian cancer cells but plays an active role in modulating the immune landscape.
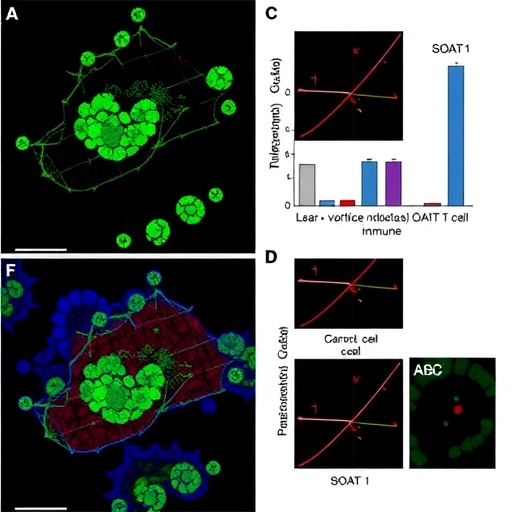
One of the fundamental aspects of the immune response in cancer is the activity of CD8+ T cells, which are cytotoxic lymphocytes tasked with identifying and destroying malignant cells. However, their effectiveness can be significantly hindered by signals from the tumor microenvironment. The authors hypothesize that SOAT1 influences lipid metabolism in ovarian cancer cells, thereby altering how these cells interact with CD8+ T cells. Their findings suggest that targeting SOAT1 may enhance the activity of these immune cells, providing a potential therapeutic avenue to reinvigorate anti-tumor immunity.
The study utilizes a range of experimental methodologies, including in vitro cell culture systems and in vivo mouse models, to dissect the role of SOAT1. By manipulating SOAT1 expression in ovarian cancer cell lines, the team was able to demonstrate distinct effects on CD8+ T cell activation and proliferation. The results indicate that SOAT1 regulates lipid composition within the tumor, which subsequently influences the expression of immunomodulatory molecules, further affecting the tumor-immune interaction.
The research is particularly timely; there has been a surge in interest surrounding metabolic pathways in cancer. While studies commonly focus on glycolysis and oxidative phosphorylation, the implications of lipid metabolism are often overlooked. This study emphasizes the need to broaden our understanding of cancer metabolism by including lipid metabolic enzymes like SOAT1. The findings contribute to a more nuanced picture of how cancer cells rewire metabolic pathways to not only support their own survival but also to manipulate immune responses.
In addition to providing evidence for the role of SOAT1 in ovarian cancer, this research raises important questions about the broader impact of lipid metabolism on tumor immunology. For instance, could modulation of lipid pathways represent a novel strategy to boost the efficacy of immunotherapies? The potential for combining targeted therapies with immunotherapeutic approaches is enormous, and understanding the interplay between these modalities is essential.
Beyond the laboratory insights, the implications of this research could reverberate throughout clinical practice. The identification of SOAT1 as a critical regulator of immune response could lead to the development of novel biomarkers for ovarian cancer patients, aiding in predictions of treatment responses and outcomes. More importantly, targeting SOAT1 in conjunction with existing therapies may enhance the overall efficacy, potentially leading to improved survival rates for patients battling this notorious disease.
While the journey from bench to bedside is fraught with challenges, the findings presented in this study underscore a vital step forward. The collaborative efforts of researchers across disciplines are crucial for translating these discoveries into therapeutic interventions. A multidisciplinary approach, integrating insights from molecular biology, immunology, and pharmacology, is essential for devising novel strategies that can effectively target the unique metabolic landscapes of tumors.
The study also sparks discussions about the potential for combination therapies that target both cancer metabolism and the immune system simultaneously. Such strategies could be particularly effective for tumors like ovarian cancer that exhibit substantial heterogeneity. Furthermore, ongoing clinical trials could offer insights into how modulation of lipid metabolism may enhance the outcomes of existing immunotherapies, driving forward the next generation of cancer treatments.
As the landscape of cancer therapy evolves, the integration of findings such as those from He et al. into clinical settings becomes increasingly relevant. The prospect of developing targeted therapies against SOAT1 not only opens new avenues for research but may also offer hope for patients facing challenging diagnoses. Ultimately, understanding the intricate networks that govern tumor immunity remains a promising frontier in cancer research.
In conclusion, the research on SOAT1’s role in mediating immune responses within ovarian cancer cells stands as a beacon of innovation in oncology. By uncovering the connections between lipid metabolism and immune modulation, this study paves the way for future explorations into therapeutic strategies that could refine how we combat ovarian and potentially other cancers. As science progresses, the hope remains that such discoveries will translate into actionable insights capable of improving patient outcomes and enriching the arsenal against cancer.
Subject of Research: The role of SOAT1 in ovarian cancer cell lipid metabolism and its influence on immune response mediated by CD8+ T cells.
Article Title: SOAT1 in ovarian cancer cells regulates immune response mediated by CD8+ T cells.
Article References:
He, J., Siu, M.K., Long, R. et al. SOAT1 in ovarian cancer cells regulates immune response mediated by CD8+ T cells.
J Ovarian Res 18, 273 (2025). https://doi.org/10.1186/s13048-025-01832-x
Image Credits: AI Generated
DOI: https://doi.org/10.1186/s13048-025-01832-x
Keywords: SOAT1, ovarian cancer, CD8+ T cells, immune response, lipid metabolism, cancer immunotherapy, tumor microenvironment.