In an illuminating advancement within immunology and cellular biology, researchers have unveiled intricate mechanisms through which Notch signaling orchestrates the fate decisions of human peripheral blood monocyte trilineage progenitors in the context of inflammation. This pioneering study not only deepens our understanding of hematopoietic lineage commitment but also opens promising avenues for therapeutic strategies targeting immune modulation in inflammatory diseases.
The Notch signaling pathway, a highly conserved cell communication system, is well-known for its pivotal role in determining cell differentiation and fate across various tissues. Its involvement in hematopoiesis, particularly in the regulation of progenitor cells that give rise to diverse myeloid lineages, has garnered increasing interest. However, a detailed exploration of its influence on monocyte progenitors, especially under inflammatory stimuli, had remained elusive until now.
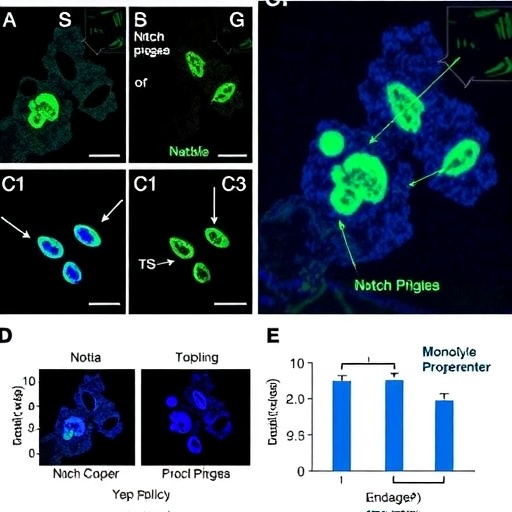
Researchers focused on trilineage progenitors derived from human peripheral blood monocytes, which possess the remarkable capacity to differentiate into three distinct effector cell types: macrophages, dendritic cells, and osteoclasts. These cell types are integral players in immune defense, antigen presentation, and bone remodeling, respectively. Understanding the cues that drive progenitors toward one lineage or another under inflammatory conditions is critical for manipulating immune responses and attenuating pathological processes.
Employing a suite of sophisticated molecular and cellular techniques, the study meticulously dissected the role Notch signaling exerts when progenitors encounter inflammatory cytokines and environmental stressors. The researchers activated and inhibited components of the Notch pathway, observing consequent changes in gene expression, surface marker profiles, and functional capacities of differentiating cells. This comprehensive approach shed light on the dynamic interplay between external inflammatory cues and intrinsic Notch-mediated regulatory mechanisms.
A salient discovery of this investigation was the identification of distinct Notch-dependent transcriptional signatures that bias progenitor commitment towards macrophage or dendritic cell lineages. Under inflammatory conditions, heightened Notch activity preferentially steered progenitors to adopt macrophage phenotypes characterized by enhanced phagocytic and pro-inflammatory functions. Conversely, attenuation of Notch signaling skewed differentiation in favor of dendritic cells, which are vital for antigen presentation and activation of adaptive immunity.
Intriguingly, the study revealed that Notch signaling also inhibits osteoclastogenesis from monocyte progenitors in inflamed environments, suggesting a protective mechanism against pathological bone resorption commonly observed in chronic inflammatory diseases such as rheumatoid arthritis. This nuanced regulation underscores Notch’s role as a multifunctional gatekeeper balancing immune defense and tissue homeostasis.
Delving deeper into molecular pathways, the team elucidated that Notch signaling modulates key transcription factors including NF-κB, IRF8, and PU.1, which are instrumental in lineage specification. These factors orchestrate gene networks that define terminal differentiation programs and functional phenotypes. The crosstalk between Notch and these transcriptional regulators represents a sophisticated regulatory nexus modulating progenitor plasticity.
Another pivotal facet of the study involved the temporal dynamics of Notch activation. Researchers demonstrated that early versus late activation of Notch signals yields divergent differentiation outcomes, emphasizing the importance of signal timing in hematopoietic programming. Such temporal control mechanisms could be exploited to fine-tune immune responses for therapeutic benefit.
Furthermore, the findings implicate inflammatory cytokines such as TNF-α and IL-6 as modulators of Notch receptor and ligand expression on progenitor cells, thereby integrating extrinsic inflammatory signals with intrinsic differentiation programs. This interface constitutes an adaptive regulatory loop whereby systemic inflammation directly influences progenitor cell fate via Notch pathways.
Implications of these insights are profound, especially for designing targeted immunotherapies. By manipulating Notch signaling components within monocyte progenitors, it may be possible to recalibrate immune responses in diseases characterized by dysregulated inflammation and aberrant myeloid cell function, including autoimmune disorders, chronic infections, and cancer.
Moreover, the selective inhibition of Notch pathways to prevent excess osteoclast formation could herald new treatments for inflammatory bone loss, offering a dual benefit of immune modulation and preservation of skeletal integrity. The translational potential of these findings positions Notch signaling as a promising target in the development of next-generation immunomodulators.
This study also underscores the critical importance of studying human cells within physiologically relevant inflammatory milieus, moving beyond animal models to capture the complexity and heterogeneity of human immune regulation. Such approaches are essential for bridging the gap between bench research and clinical application.
While the results provide compelling evidence for Notch’s multifaceted roles, the authors acknowledge limitations, including the need for in vivo validation and exploration of Notch interactions with other signaling pathways such as Wnt and Hedgehog. Future research is poised to untangle these complex networks, offering richer insights into immune progenitor biology.
In conclusion, this groundbreaking investigation delineates how Notch signaling dynamically governs the fate of human peripheral blood monocyte trilineage progenitors under inflammatory conditions, finely tuning the balance between macrophage, dendritic cell, and osteoclast lineages. These findings invigorate the field with fresh mechanistic understanding and lay a robust foundation for harnessing Notch pathways in therapeutic innovation.
As chronic inflammatory conditions continue to impose significant health burdens worldwide, the modulation of progenitor cell fate through Notch offers a beacon of hope. The ability to direct immune cell differentiation with precision could revolutionize treatment paradigms, enabling tailored interventions that restore immune equilibrium without broad immunosuppression.
The research community and clinical practitioners alike will keenly watch forthcoming studies that build upon these seminal discoveries. By integrating molecular insights with clinical needs, the path toward transformative immune therapies may be rapidly accelerated, fulfilling the promise of precision medicine.
Continued investment in decoding cell signaling mechanisms and their contextual dependencies remains paramount. The elucidation of Notch’s role herein exemplifies the power of fundamental research to illuminate complex biological systems and inspire novel therapeutic strategies.
This study handles complexities of immune differentiation with elegant experimental strategies, offering clarity into a previously obscure regulatory axis. Its publication marks a significant milestone in both immunology and cell biology, likely to galvanize further inquiries and technological advancements.
The intersection of Notch signaling and inflammatory microenvironments unveiled by this research reflects the evolving landscape of hematopoietic science, one where signaling pathways are viewed not in isolation but as integrated systems influencing disease outcomes and clinical opportunities alike.
Subject of Research: The influence of Notch signaling on the lineage commitment of human peripheral blood monocyte trilineage progenitors under inflammatory conditions.
Article Title: Effects of Notch signaling on the lineage commitment of human peripheral blood monocyte trilineage progenitor under inflammatory conditions.
Article References:
Aničić, S., Filipović, M., Krešić, I. et al. Effects of Notch signaling on the lineage commitment of human peripheral blood monocyte trilineage progenitor under inflammatory conditions. Cell Death Discov. 11, 519 (2025). https://doi.org/10.1038/s41420-025-02807-z
Image Credits: AI Generated
DOI: 10 November 2025