In a notable advancement in breast cancer treatment, researchers have reported a compelling case of metastatic HER2-positive breast cancer complicated by leptomeningeal carcinomatosis, treated with the antibody-drug conjugate trastuzumab deruxtecan. This case exemplifies the therapeutic potential of targeted agents in central nervous system (CNS) metastases, an area historically fraught with clinical challenges due to the blood-brain barrier’s limitations and aggressive tumor biology.
Leptomeningeal carcinomatosis (LMC) represents a devastating complication where cancer cells infiltrate the leptomeninges, the thin membranes enveloping the brain and spinal cord. This condition often heralds a dismal prognosis, with neurological decline and survival measured in mere months. The rarity of effective systemic therapies that can penetrate the CNS underscores the urgency for innovative approaches, making this case report particularly groundbreaking.
The patient, a 37-year-old female initially diagnosed with HER2-positive breast cancer, underwent standard therapeutic protocols, including surgery, chemotherapy, and HER2-targeted therapy. Despite initial remission, she experienced a relapse involving the CNS two years later, manifesting with neurological impairments indicative of leptomeningeal involvement. This progression exemplifies the aggressive nature and therapeutic resistance characteristic of CNS metastatic burden.
Recognizing the paucity of approved treatments for LMC and harnessing emerging data, clinicians opted for off-label administration of trastuzumab deruxtecan. This agent, an antibody-drug conjugate (ADC), couples the HER2-targeting monoclonal antibody trastuzumab with a topoisomerase I inhibitor payload, enabling targeted delivery of cytotoxic chemotherapy directly to HER2-expressing cancer cells. The design theoretically enhances tumor selectivity while sparing normal tissues.
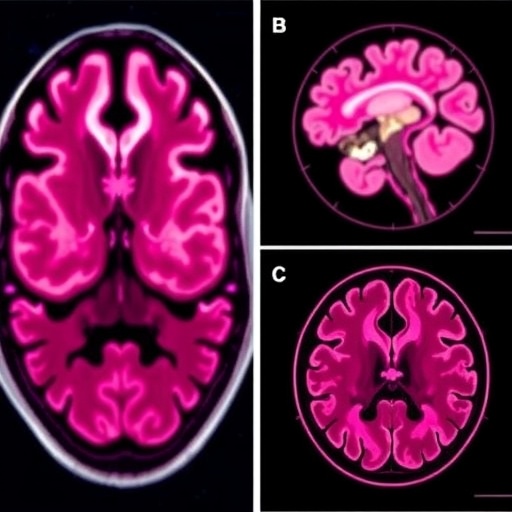
Following three cycles of treatment, the patient exhibited marked clinical improvements, evidenced by alleviation of neurological symptoms and regained functional independence. Magnetic resonance imaging (MRI) revealed appreciable reduction in tumor bulk within both the leptomeningeal spaces and brain parenchyma. Vasogenic edema and sulcal effacement, previously noted around the metastatic lesions, also demonstrated regression, highlighting the agent’s effectiveness in controlling aggressive CNS disease components.
Maintenance therapy was sustained for over two years, during which the CNS disease remained radiographically stable. This prolonged disease control period is noteworthy given the historically rapid progression typical of leptomeningeal carcinomatosis. The tolerability profile was acceptable, with manageable adverse effects, supporting the feasibility of extended trastuzumab deruxtecan administration in such complex cases.
This case underscores the importance of integrating biological insights, advanced imaging, and multidisciplinary care in managing refractory metastatic breast cancer with CNS involvement. The ability of trastuzumab deruxtecan to traverse the blood-brain barrier and deliver potent cytotoxic payloads offers a promising therapeutic avenue, potentially shifting paradigms in the management of HER2-positive CNS metastases.
The underlying mechanism by which trastuzumab deruxtecan exerts its effects involves selective binding to HER2-expressing tumor cells, internalization of the ADC, and intracellular release of the cytotoxic topoisomerase I inhibitor. This approach counters tumor heterogeneity and resistance mechanisms by delivering high concentrations of chemotherapy directly to malignant cells while limiting systemic exposure.
Clinically, this report advocates for personalized oncology strategies, emphasizing the need to tailor treatments dynamically as disease biology evolves. It also highlights the potential benefits of off-label therapeutic uses guided by molecular tumor profiling and preclinical data, especially in conditions lacking standardized care protocols.
The broader implications of this case extend to ongoing clinical research efforts evaluating ADCs and other novel agents for CNS metastatic diseases. Expanding the arsenal of effective treatments against leptomeningeal and brain metastases can improve survival outcomes and quality of life for patients facing these formidable complications.
Moreover, the interdisciplinary collaboration among oncologists, neurologists, radiologists, and pharmacologists was integral to the successful management of this case. Diagnostic precision through contrast-enhanced MRI facilitated accurate disease staging and response monitoring, illustrating the critical role of imaging biomarkers in contemporary oncology.
While this case report is singular, it holds significant promise for informing larger trials and guiding clinical decisions in similar patient populations. Continued investigation into optimal dosing, sequencing, and combination strategies with trastuzumab deruxtecan and other ADCs is warranted to maximize therapeutic benefit and overcome resistance.
In conclusion, the successful application of trastuzumab deruxtecan in treating metastatic HER2-positive breast cancer with leptomeningeal carcinomatosis marks a significant step forward. It signals a potential new era in which targeted ADC therapies become standard components in the management of CNS metastatic breast cancer, transforming previously terminal conditions into chronic, manageable diseases.
Subject of Research: People
Article Title: Metastatic breast cancer with leptomeningeal carcinomatosis treated with trastuzumab deruxtecan – a case report
News Publication Date: 9 October 2025
Web References: http://dx.doi.org/10.18632/oncoscience.631
Image Credits: Copyright: © 2025 Martins et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0)
Keywords: cancer, breast cancer, leptomeningeal carcinomatosis, HER2 positive, antibody-drug conjugate, trastuzumab deruxtecan