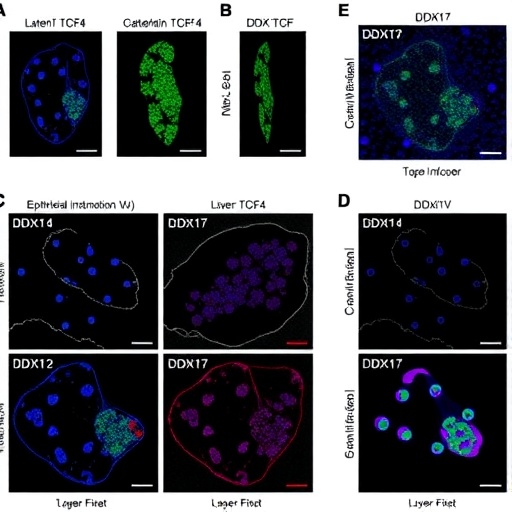
A groundbreaking study from the Research Center for Preclinical Medicine at Southwest Medical University unveils new insights into the molecular drivers behind hepatocellular carcinoma (HCC) metastasis, highlighting the critical role of the DEAD-box RNA helicase DDX17. HCC, notorious for high morbidity and mortality rates worldwide, especially suffers from the aggressive nature of its metastatic spread, which dramatically diminishes patient survival outcomes. This novel research delineates a previously uncharacterized feedback loop involving DDX17, β-catenin, and TCF4 that orchestrates epithelial-mesenchymal transition (EMT), a pivotal event enabling HCC cells to migrate, invade, and colonize distant tissues.
By analyzing tumor samples, the team first established that DDX17 expression is markedly elevated in HCC tissues compared to adjacent normal liver tissues, correlating positively with tumor aggressiveness, invasion potential, and advanced clinical staging. In vitro assays confirmed that artificially reducing DDX17 levels curtailed the motility and invasiveness of cultured HCC cells, while its overexpression amplified these malignant traits. This bidirectional experimental confirmation establishes DDX17 not just as a marker but as a functional instigator of metastatic behavior in liver cancer.
Pivoting to in vivo models, the researchers employed an orthotopic nude mouse model of HCC, where they demonstrated that DDX17 silencing substantially tempered metastatic dissemination in living organisms. These findings solidify the translational relevance of DDX17, emphasizing its indispensable role in driving HCC metastasis beyond cell culture systems, and moving closer to clinical applicability.
At the molecular level, the study intricately explores how DDX17 influences EMT, a key program wherein epithelial tumor cells acquire mesenchymal properties, gaining enhanced motility which underpins metastatic competence. The research reveals that DDX17 facilitates the downregulation of epithelial markers such as E-cadherin while concomitantly upregulating mesenchymal markers including N-cadherin, Snail, and Vimentin. This phenotypic switch aligns with enhanced migratory and invasive capabilities traditionally associated with EMT processes.
Diving deeper into the mechanistic pathways, the study sheds light on the transcriptional regulation governing DDX17. The transcription factor TCF4, known for partnering with β-catenin in canonical Wnt signaling, was found to bind directly to the DDX17 promoter region, augmenting its transcriptional activity. This direct regulatory input designates TCF4 as a critical upstream activator of DDX17 expression in HCC cells, positioning it within a tightly controlled oncogenic axis.
Crucially, DDX17 itself was shown to promote the nuclear translocation of β-catenin, a multifunctional protein with dual roles in cell adhesion and gene transcription regulation. This translocation relies on the integrity of DDX17’s helicase domain, suggesting that its enzymatic activity is vital for modulating β-catenin localization. Once in the nucleus, β-catenin partners with TCF4 to further stimulate DDX17 gene expression, thus completing a positive feedback loop that sustains and amplifies the metastatic phenotype.
This β-catenin/TCF4/DDX17 loop represents an intricate regulatory circuitry that not only perpetuates DDX17 overexpression but also reinforces EMT, resulting in enhanced migration and invasion capabilities of HCC cells. Importantly, disruption of any component within this loop significantly attenuates the invasive potential of cancer cells, underscoring its functional indispensability.
Such a feedback mechanism highlights a vulnerable node in HCC metastatic cascade that could be exploited therapeutically. Targeting the helicase activity of DDX17 or its interaction interfaces within this loop presents a promising strategy to arrest or slow down HCC progression, potentially improving patient prognoses where current treatments are inadequate.
In their statements, Dr. Junjiang Fu remarked, “Our study reveals a novel DDX17-driven pathway in HCC metastasis, highlighting its potential as a therapeutic target to halt malignant progression.” Dr. Chaoxiang Lv further emphasized that “The β-catenin/TCF4/DDX17 feedback loop provides a mechanistic foundation for understanding HCC dissemination and opens avenues for targeted intervention,” signaling the translational impact of their findings.
These findings resonate strongly within the cancer research community, as they integrate RNA helicase biology with canonical Wnt signaling and EMT regulation, weaving together disparate molecular threads into a unified model of HCC metastasis. By bridging these pathways, the research not only advances fundamental understanding but also pioneers prospects for innovative drug development.
As hepatocellular carcinoma remains a formidable clinical challenge, uncovering molecular vulnerabilities like the DDX17-driven feedback loop represents a beacon of hope. Moving forward, effort will likely focus on developing inhibitors or modulators of this cascade, alongside biomarker development for identifying high-risk patients who may benefit most from such targeted therapies.
The study, published in the esteemed journal MedComm – Oncology, exemplifies the synergy between molecular biology and translational medicine, setting a new paradigm for tackling liver cancer metastasis through the lens of DDX17’s oncogenic functions.
Subject of Research: Molecular mechanisms underpinning DDX17-driven hepatocellular carcinoma metastasis.
Article Title: β-Catenin/TCF4 Is Required for DDX17-Induced Epithelial–Mesenchymal Transition and Metastasis in Hepatocellular Carcinoma
News Publication Date: 14-Sep-2025
Web References: DOI: 10.1002/mog2.70039
Image Credits: Junjiang Fu and Chaoxiang Lv
Keywords: DDX17, hepatocellular carcinoma, metastasis, epithelial-mesenchymal transition, β-catenin, TCF4, feedback loop, RNA helicase, tumor invasion, molecular mechanisms