In an unprecedented breakthrough illuminating the complex cellular interplay driving sepsis-induced liver damage, researchers have identified a novel molecular pathway that could fundamentally shift therapeutic strategies against this devastating condition. The study, led by Sun, Wu, Liu, and colleagues, dives deep into the intricate mechanisms modulating Kupffer cell polarization—the liver’s resident macrophages—and how these changes influence the inflammatory cascade typical of sepsis. Central to their findings is the role of the YTH domain N6-methyladenosine RNA binding protein F1 (YTHDF1), which has emerged as a critical regulator attenuating hepatic injury by promoting anti-inflammatory phenotypes within Kupffer cells.
Sepsis remains a global medical emergency marked by a dysregulated immune response to infection, often culminating in multiple organ failures, with liver dysfunction being a significant predictor of patient mortality. The liver’s innate immune cells, Kupffer cells, can polarize into distinct phenotypes that either exacerbate inflammatory damage (M1) or facilitate tissue repair and resolution (M2). Modulating this phenotypic switch towards M2 polarization is hailed as a promising therapeutic target, yet the molecular underpinnings dictating this transition have remained elusive. This study propels the field forward by unveiling the YTHDF1-mediated molecular circuitry that orchestrates this critical polarization balance under septic conditions.
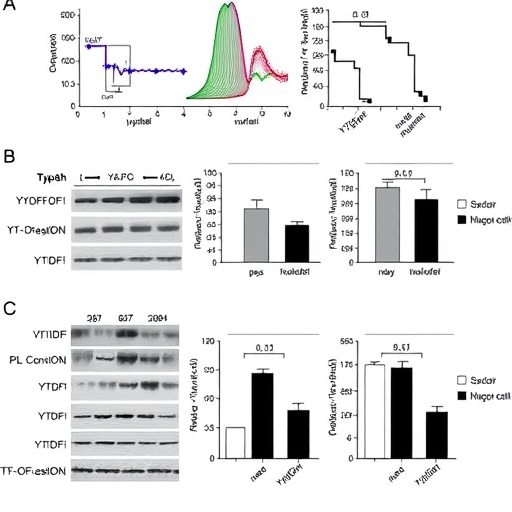
Employing a combination of cecal ligation and puncture (CLP) mouse models which mimic sepsis-induced liver injury, alongside LPS-stimulated macrophage cell lines, the research team executed comprehensive histological assessments to characterize hepatic tissue damage. Flow cytometry analyses further dissected the expression profiles of macrophage markers, distinguishing pro-inflammatory M1 (iNOS-positive) from anti-inflammatory M2 (Arg1-positive) states in Kupffer cells. Through western blotting, quantified protein expression levels highlighted the dynamic shifts in YTHDF1, KLF2, and VSIG4—key molecular protagonists in this newly delineated pathway.
At the heart of the study’s molecular narrative is Krüppel-like factor 2 (KLF2), a zinc finger transcription factor previously recognized for its role in vascular homeostasis but now shown to be pivotal in regulating Kupffer cell function during sepsis. KLF2 expression was markedly reduced in sepsis-induced liver tissue and macrophages challenged by LPS, correlating with heightened inflammatory states. However, when KLF2 was upregulated, the researchers observed a robust increase in VSIG4 transcription—a complement receptor associated with immunosuppressive and anti-inflammatory responses in macrophages—thereby tipping Kupffer cells towards the M2 phenotype and fostering hepatocyte protection.
Strikingly, the modulation of KLF2 expression was found to be intimately tied to post-transcriptional RNA modifications. YTHDF1, an m6A “reader” protein that recognizes and binds N6-methyladenosine-marked RNA, was shown to govern the translation of KLF2 by regulating its m6A methylation status. This axis not only stabilizes KLF2 mRNA but enhances its protein synthesis, reinforcing the anti-inflammatory switch in Kupffer cells. Through chromatin immunoprecipitation (ChIP), RNA immunoprecipitation (RIP), and dual luciferase reporter assays, the researchers mapped the precise interactions underpinning this regulatory cascade, providing a molecular blueprint of YTHDF1’s control over KLF2 and consequential VSIG4 expression.
The functional implications of manipulating this axis were profound. Overexpression of either YTHDF1 or KLF2 in Kupffer cells effectively skewed macrophage polarization toward the M2 subtype, which in turn markedly alleviated liver injury in CLP-operated mice. This attenuation of hepatic damage was evidenced by histological improvements, reduced hepatocyte apoptosis, and diminished inflammatory markers, collectively underscoring the therapeutic potential of targeting the YTHDF1-KLF2-VSIG4 pathway in sepsis care. These findings present a paradigm shift from broad-spectrum anti-inflammatory treatments toward precise molecular interventions that harness endogenous immune modulation.
Beyond the immediate translational value, this study also highlights the pivotal role of epitranscriptomic modifications—specifically, m6A methylation—in governing immune cell behavior during critical illness. YTHDF1’s function as a selective mRNA methylation reader elaborates a new layer of gene expression regulation in the context of sepsis, inviting deeper exploration into how RNA modifications can be exploited for therapeutic gain. This aligns with growing evidence in immunology that epitranscriptomic modulation governs cellular plasticity, potentially offering refined control over immune responses.
Moreover, the identification of VSIG4 as a downstream effector of KLF2 provides additional insight into the immunoregulatory mechanisms within the liver microenvironment. VSIG4 has emerged as a negative regulator of macrophage activation, capable of dampening pro-inflammatory signaling pathways. By establishing the transcriptional link between KLF2 and VSIG4, the study delineates a cohesive chain of regulatory events that culminate in Kupffer cell anti-inflammatory polarization. This axis, therefore, represents not just a biomarker but a potential therapeutic target to recalibrate immune balance in sepsis.
In the broader context of sepsis research, these findings are particularly vital given the persistent challenge of reducing high mortality and morbidity associated with this syndrome. The liver’s central role as an immunologic and metabolic hub means that protecting hepatic function during septic insults can substantially improve patient prognosis. By elucidating a mechanistic axis capable of reprogramming resident macrophages to a reparative state, the study contributes to the crucial endeavor of designing targeted, mechanism-driven therapies rather than relying on supportive care alone.
Crucially, the experiments also underscored the reversibility of Kupffer cell polarization under septic conditions, mediated through molecular interventions involving YTHDF1 and KLF2. Such reversibility is a promising indication that even during the acute phases of sepsis-induced liver injury, therapeutic modulation of this pathway could restore homeostasis and mitigate irreversible tissue damage. This dynamic plasticity challenges prior notions of fixed immune cell states in sepsis, opening new avenues for temporal therapeutic application.
Furthermore, the employment of sophisticated molecular techniques such as flow cytometry, western blotting, ChIP, RIP, and luciferase assays attest to the study’s rigorous approach in verifying the multi-tiered interactions between RNA-binding proteins and transcription factors. This methodological robustness lends substantial credibility to the findings and deepens the scientific community’s understanding of RNA epigenetics as a pivotal player in immune responses during sepsis.
The translational significance of this research extends beyond sepsis, as macrophage polarization plays a critical role in various inflammatory and autoimmune diseases, as well as in cancer biology. The demonstration that modulating RNA methylation via factors like YTHDF1 can influence macrophage phenotype suggests potential therapeutic applications across a spectrum of diseases where inflammation drives pathology.
In conclusion, this groundbreaking study not only enriches our understanding of the molecular crosstalk within hepatic immune cells during sepsis but also defines YTHDF1 and its downstream targets KLF2 and VSIG4 as promising therapeutic nodes. By harnessing the epitranscriptomic machinery that fine-tunes macrophage polarization, this work paves the way for targeted therapies aimed at mitigating liver damage and improving outcomes in septic patients. Future clinical investigations will be essential to translate these molecular insights into viable interventions, potentially transforming the management of sepsis worldwide.
As research progressively uncovers the subtleties of immune regulation at the RNA level, the findings presented here reinforce the notion that the epitranscriptome is an untapped frontier ripe for exploration and exploitation in the fight against critical illness. The YTHDF1-KLF2-VSIG4 axis promises to be a focal point in the evolving landscape of immunotherapy, molecular medicine, and precision healthcare targeting sepsis and beyond.
Subject of Research: The study focuses on elucidating the molecular mechanisms by which YTHDF1 regulates Kupffer cell polarization via the KLF2/VSIG4 axis to mitigate sepsis-induced liver injury.
Article Title: YTHDF1 mediates KLF2/VSIG4 axis to regulate Kupffer cell polarization to alleviate sepsis-induced liver injury.
Article References:
Sun, N., Wu, Y., Liu, B. et al. YTHDF1 mediates KLF2/VSIG4 axis to regulate Kupffer cell polarization to alleviate sepsis-induced liver injury. Genes Immun (2025). https://doi.org/10.1038/s41435-025-00367-x
Image Credits: AI Generated
DOI: 07 November 2025
Keywords: Sepsis, liver injury, Kupffer cells, macrophage polarization, YTH domain N6-methyladenosine RNA binding protein F1 (YTHDF1), Krüppel-like factor 2 (KLF2), VSIG4, m6A methylation, epitranscriptomics, inflammation, hepatic immunology