In a healthy human body, tissue growth and development are coordinated by many different mechanisms. Within our bodies, these mechanisms regulate the healthy growth of cells, limit their size and number, and control the timing of cell death through apoptosis. However, when these regulatory pathways are altered, or break down, cell growth and proliferation may increase beyond what is safe and this can lead to cancer. One critical cell growth regulatory mechanism is the Hippo signaling pathway. This pathway regulates the expression of several genes that control cell proliferation and apoptosis via the transcription coactivators YAP/TAZ. Dysregulation of this pathway is commonly seen in several different cancers including head and neck squamous cell carcinoma (HNSCC).
One promising anti-tumor drug is indisulam, a cell cycle inhibitor that inhibits the expression of cell proliferation genes and triggers aberrant mRNA splicing by degrading the RNA-binding protein (RBM39) causing cell death. It has shown promise both in cell cultures and in animal studies. However, clinical trials have shown fewer clear-cut results. Scientists from Hiroshima University Hospital have been researching the reasons behind this resistance to indisulam.
“Clinical trials of indisulam failed to show good response in solid cancer patients but the mechanism of the resistance has not been solved. I wondered if YAP/RBM39 interaction may be involved in the resistance mechanism of indisulam,” said Toshinori Ando, senior lecturer at the Center of Oral Clinical Examination, Hiroshima University Hospital.
In fact, they found “that YAP interacts with RBM39 and confers resistance against indisulam, which is the critical obstruction to be solved for future clinical usage of indisulam,” Ando said. Their research was published on July 15 in Oncogenesis.
A signaling pathway is a series of chemical reactions that are activated by a signal, often environmental. Once begun, each reaction within the pathway is activated in series ending up with the activation or inactivation of a specific cellular function. This allows the body to react to the presence or absence of specific chemicals, such as hormones, by activating or deactivating transcription of certain genes depending on what the cell needs at that moment. The Hippo signalling pathway is a critical pathway that can deactivate the transcription of genes that control cell proliferation by inactivating YAP/TAZ.
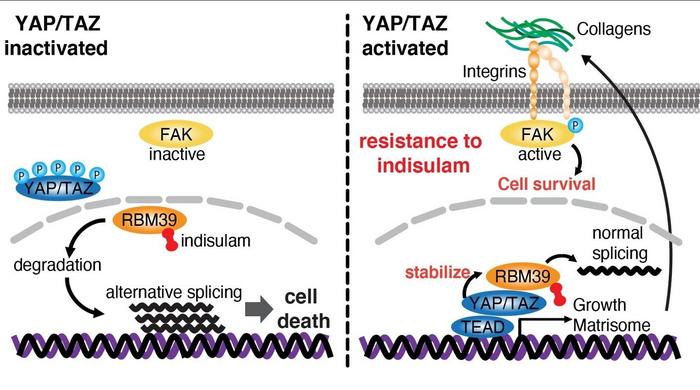
The anti-tumor drug indisulam acts by degrading RBM39, a protein that binds to transcription factors, which enhances transcription as well as regulating the mRNA splicing that is integral to the process of gene transcription. By degrading RBM39, indisulam interferes with RBM39’s normal function causing changes in splicing leading to cell death thereby reducing the cell proliferation that can lead to tumor formation.
The Hiroshima University researchers focused on the reactions within the nucleus between the YAP/TAZ transcriptional coactivators and RBM39 to understand what exactly was occurring between them. They also investigated what happens when indisulam was added to determine what was causing the resistance to the anti-tumor drug.
Using proteome analysis, the group identified RBM39’s interactions with YAP/TAZ. They also found evidence that it does promote YAP/TAZ’s transcriptional activity. When indisulam was added it inactivated a focal adhesion kinase (FAK), an enzyme which is very important for cell survival and which caused degradation in RBM39, thereby shutting down the transcription of the cell proliferation genes.
Interestingly, they discovered that the root of indisulam resistance was the activation of YAP/TAZ. When activated it delayed indisulam’s ability to induce the degradation of RBM39 and it reactivated the FAK, thereby reducing the drug’s effectiveness. Activation of YAP/TAZ conferred resistance both in vivo, in cell cultures and in vitro, in mice.
“In this study, we identified RBM39 as a novel YAP/TAZ interactor in the nucleus. Notably, we demonstrated that YAP/TAZ interacts with RBM39 to confer resistance to indisulam. Our findings decipher the resistance mechanism of indisulam, which may aid the development of a novel therapeutic approach for patients with solid cancers including HNSCC,” Ando said.
Going forward, their research will be focusing on “further mechanism of interaction between RBM39 and YAP, thereby developing a new drug targeting the interaction for the patients with solid cancers including HNSCC,” Ando said.
Other contributors include Nanako Kataoka, Tomoaki Shintani and Mikihito Kajiya from the Center of Oral Clinical Examination, Hiroshima University Hospital; Kento Okamoto, Yume Ueda and Souichi Yanamoto from the Department of Oral Oncology, Graduate School of Biomedical and Health Sciences, Hiroshima University; and Mutsumi Miyauchi from the Department of Oral and Maxillofacial Pathobiology, Graduate School of Biomedical and Health Sciences, Hiroshima University.
This work was supported by JSPS JP20K18477, JP22H03275, and JP24K02645, the “Nozomi h foundation” from the Hiroshima University Foundation, and the JST HIRAKU-Global program.

Credit: Toshinori Ando, Center of Oral Clinical Examination, Hiroshima University Hospital
In a healthy human body, tissue growth and development are coordinated by many different mechanisms. Within our bodies, these mechanisms regulate the healthy growth of cells, limit their size and number, and control the timing of cell death through apoptosis. However, when these regulatory pathways are altered, or break down, cell growth and proliferation may increase beyond what is safe and this can lead to cancer. One critical cell growth regulatory mechanism is the Hippo signaling pathway. This pathway regulates the expression of several genes that control cell proliferation and apoptosis via the transcription coactivators YAP/TAZ. Dysregulation of this pathway is commonly seen in several different cancers including head and neck squamous cell carcinoma (HNSCC).
One promising anti-tumor drug is indisulam, a cell cycle inhibitor that inhibits the expression of cell proliferation genes and triggers aberrant mRNA splicing by degrading the RNA-binding protein (RBM39) causing cell death. It has shown promise both in cell cultures and in animal studies. However, clinical trials have shown fewer clear-cut results. Scientists from Hiroshima University Hospital have been researching the reasons behind this resistance to indisulam.
“Clinical trials of indisulam failed to show good response in solid cancer patients but the mechanism of the resistance has not been solved. I wondered if YAP/RBM39 interaction may be involved in the resistance mechanism of indisulam,” said Toshinori Ando, senior lecturer at the Center of Oral Clinical Examination, Hiroshima University Hospital.
In fact, they found “that YAP interacts with RBM39 and confers resistance against indisulam, which is the critical obstruction to be solved for future clinical usage of indisulam,” Ando said. Their research was published on July 15 in Oncogenesis.
A signaling pathway is a series of chemical reactions that are activated by a signal, often environmental. Once begun, each reaction within the pathway is activated in series ending up with the activation or inactivation of a specific cellular function. This allows the body to react to the presence or absence of specific chemicals, such as hormones, by activating or deactivating transcription of certain genes depending on what the cell needs at that moment. The Hippo signalling pathway is a critical pathway that can deactivate the transcription of genes that control cell proliferation by inactivating YAP/TAZ.
The anti-tumor drug indisulam acts by degrading RBM39, a protein that binds to transcription factors, which enhances transcription as well as regulating the mRNA splicing that is integral to the process of gene transcription. By degrading RBM39, indisulam interferes with RBM39’s normal function causing changes in splicing leading to cell death thereby reducing the cell proliferation that can lead to tumor formation.
The Hiroshima University researchers focused on the reactions within the nucleus between the YAP/TAZ transcriptional coactivators and RBM39 to understand what exactly was occurring between them. They also investigated what happens when indisulam was added to determine what was causing the resistance to the anti-tumor drug.
Using proteome analysis, the group identified RBM39’s interactions with YAP/TAZ. They also found evidence that it does promote YAP/TAZ’s transcriptional activity. When indisulam was added it inactivated a focal adhesion kinase (FAK), an enzyme which is very important for cell survival and which caused degradation in RBM39, thereby shutting down the transcription of the cell proliferation genes.
Interestingly, they discovered that the root of indisulam resistance was the activation of YAP/TAZ. When activated it delayed indisulam’s ability to induce the degradation of RBM39 and it reactivated the FAK, thereby reducing the drug’s effectiveness. Activation of YAP/TAZ conferred resistance both in vivo, in cell cultures and in vitro, in mice.
“In this study, we identified RBM39 as a novel YAP/TAZ interactor in the nucleus. Notably, we demonstrated that YAP/TAZ interacts with RBM39 to confer resistance to indisulam. Our findings decipher the resistance mechanism of indisulam, which may aid the development of a novel therapeutic approach for patients with solid cancers including HNSCC,” Ando said.
Going forward, their research will be focusing on “further mechanism of interaction between RBM39 and YAP, thereby developing a new drug targeting the interaction for the patients with solid cancers including HNSCC,” Ando said.
Other contributors include Nanako Kataoka, Tomoaki Shintani and Mikihito Kajiya from the Center of Oral Clinical Examination, Hiroshima University Hospital; Kento Okamoto, Yume Ueda and Souichi Yanamoto from the Department of Oral Oncology, Graduate School of Biomedical and Health Sciences, Hiroshima University; and Mutsumi Miyauchi from the Department of Oral and Maxillofacial Pathobiology, Graduate School of Biomedical and Health Sciences, Hiroshima University.
This work was supported by JSPS JP20K18477, JP22H03275, and JP24K02645, the “Nozomi h foundation” from the Hiroshima University Foundation, and the JST HIRAKU-Global program.
###
About Hiroshima University
Since its foundation in 1949, Hiroshima University has striven to become one of the most prominent and comprehensive universities in Japan for the promotion and development of scholarship and education. Consisting of 12 schools for undergraduate level and 4 graduate schools, ranging from natural sciences to humanities and social sciences, the university has grown into one of the most distinguished comprehensive research universities in Japan. English website:
Journal
Oncogenesis
Article Title
YAP/TAZ interacts with RBM39 to confer resistance against indisulam
Article Publication Date
15-Jul-2024
COI Statement
The authors declare no competing interests.