Exploring new energy storage and conversion technologies is crucial for sustainable human development. Proton exchange membrane fuel cells (PEMFCs) and metal-air batteries are particularly promising due to their high energy efficiency and environmental friendliness. A key component in these technologies is the oxygen reduction reaction (ORR) catalyst. Traditionally, ORR catalysts rely on expensive platinum group metals (PGM), which are cost-prohibitive for widespread use. This has spurred interest in developing non-precious metal alternatives, such as transition metal/nitrogen-doped carbon-based materials (M–N–C). Among these, Fe–N–C catalysts, where iron is coordinated with nitrogen in the Fe–N4 configuration, are notable for their Pt-like behavior in catalyzing ORR, particularly in terms of O2 adsorption and O=O bond breaking. However, these catalysts often suffer from limited activity and rapid degradation, especially in acidic conditions, due to suboptimal adsorption energy for oxygen intermediates.

Credit: ©Science China Press
Exploring new energy storage and conversion technologies is crucial for sustainable human development. Proton exchange membrane fuel cells (PEMFCs) and metal-air batteries are particularly promising due to their high energy efficiency and environmental friendliness. A key component in these technologies is the oxygen reduction reaction (ORR) catalyst. Traditionally, ORR catalysts rely on expensive platinum group metals (PGM), which are cost-prohibitive for widespread use. This has spurred interest in developing non-precious metal alternatives, such as transition metal/nitrogen-doped carbon-based materials (M–N–C). Among these, Fe–N–C catalysts, where iron is coordinated with nitrogen in the Fe–N4 configuration, are notable for their Pt-like behavior in catalyzing ORR, particularly in terms of O2 adsorption and O=O bond breaking. However, these catalysts often suffer from limited activity and rapid degradation, especially in acidic conditions, due to suboptimal adsorption energy for oxygen intermediates.
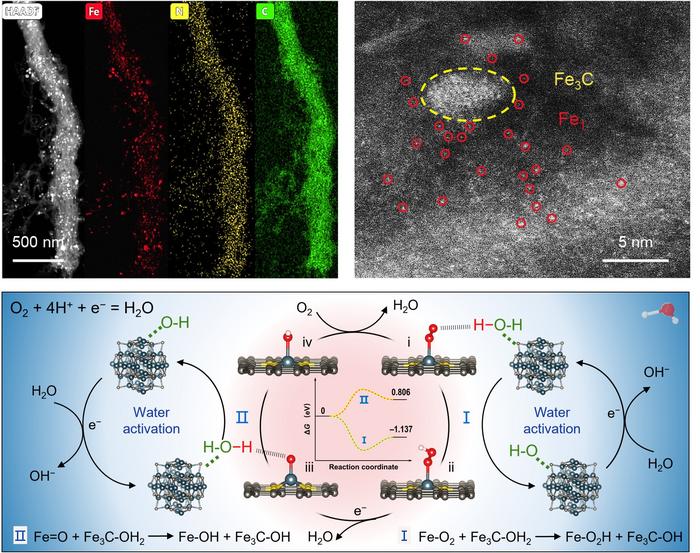
To enhance the intrinsic activity and stability of Fe-based catalysts, various strategies have been employed, including doping with heteroatoms (e.g., S, P), introducing neighboring transition metal sites (e.g., Co, Ni, Mn), and adding axial coordination groups (e.g., O, OH, Cl) at the metal center. Transition metal compounds, particularly those modifying atomically dispersed Fe–N4 sites, have shown potential in improving ORR performance in acidic media. Nonetheless, the role of water activation in the ORR process, often underappreciated, is critical. In acidic conditions, a strong electric field polarizes interfacial water molecules into an O-down configuration, disrupting hydrogen bonding with oxygenated intermediates and slowing down the proton-coupled electron transfer (PCET) step. Enhancing hydrogen bonding between these intermediates and interfacial water through effective water activation sites can significantly speed up PCET and ORR kinetics. The platinum-like electronic configuration of Fe3C makes it an effective catalytic site for water activation, improving proton generation from water and boosting ORR activity when paired with traditional Fe-N4 sites.
Imagine a catalyst that can provide performance comparable to traditional platinum metals but at a lower cost. Professor Peng’s research team has made significant progress in this direction. Their developed Fe3C-Fe1/CNT catalyst not only exhibits an excellent half-wave potential of 0.83 V in acidic media but also achieves 0.91 V in alkaline media. This means it performs exceptionally well in actual H2-O2 fuel cells and aluminum-air batteries. Through a meticulous process of treating the electrospun precursor, followed by thermal decomposition and etching, the research team created a nanomaterial with a three-dimensional network structure and excellent electron transfer capabilities. This structure not only increases the exposure of active sites but also facilitates the rapid conversion of reactants to products.
In traditional proton exchange membrane fuel cells and metal-air batteries, the oxygen reduction reaction often relies on expensive platinum-group metals. Professor Peng’s research offers a more economical and efficient alternative. This catalyst, composed of coordinated Fe and N, demonstrates excellent stability and high efficiency in both acidic and alkaline environments, which is crucial for advancing sustainable energy technologies. The article’s diagrams and data further substantiate these findings, displaying the structure and performance test results of the catalyst in detail. Through in situ Fourier-transform infrared spectroscopy and density functional theory analysis, the research team revealed the key role of Fe3C in activating water molecules and enhancing reaction rates.
Professor Peng’s team plans to further explore the application of this catalyst in other electrocatalytic reactions, hoping to bring more breakthroughs to future energy conversion technologies through continuous innovation and optimization.
###
See the article:
Fe-N co-doped carbon nanofibers with Fe3C decoration for water activation induced oxygen reduction reaction
Journal
National Science Review