Mitochondria, long celebrated as the cellular powerhouses, have emerged as pivotal arbiters of cancer progression, especially in aggressive breast cancers like triple-negative breast cancer (TNBC). Recent insights reveal that the dynamic remodeling of mitochondrial networks—through tightly regulated processes of fission, fusion, and mitophagy—is not simply a cellular housekeeping mechanism, but a critical driver of tumor metabolism, adaptability, and metastasis. As researchers unravel the intricate molecular choreography governing these mitochondrial dynamics, a new frontier emerges offering promising therapeutic interventions against formidable breast cancer subtypes.
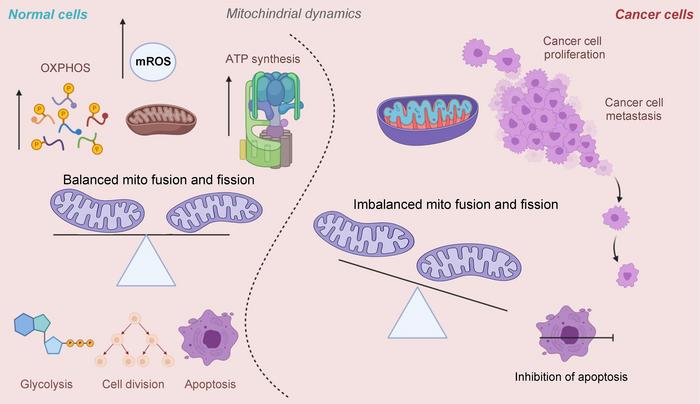
At the heart of cellular bioenergetics, mitochondrial fusion and fission must strike a delicate balance for optimal function. Fusion joins mitochondria, facilitating efficient ATP production through oxidative phosphorylation (OXPHOS) and controlling reactive oxygen species (ROS) levels. Conversely, fission fragments mitochondria, a process essential for cell division, apoptosis, and metabolic reprogramming. In normal cells, these opposing forces cooperate to maintain metabolic homeostasis. However, in cancer cells, and particularly in TNBC, this equilibrium shifts decisively toward excessive fission, fueling the malignant traits of unchecked proliferation, enhanced metastatic potential, and the maintenance of cancer stem cell-like properties.
Breast cancer exhibits profound metabolic heterogeneity, a feature most prominent in the notoriously treatment-resistant TNBC. While the historical Warburg effect posited glycolysis as the dominant energy source even in oxygen-rich environments, emerging data highlight the complexity of mitochondrial metabolism’s role in cancer biology. TNBC cells leverage fatty acid oxidation (FAO) and robust mitochondrial respiration to satisfy their heightened energetic and biosynthetic demands. Enzymes like fatty acid synthase (FASN) and ATP citrate lyase elevate de novo lipogenesis, supporting membrane biosynthesis and oncogenic signaling pathways necessary for rapid tumor expansion.
Interestingly, although primary breast tumors often rely heavily on glycolysis, metastatic lesions display increased tricarboxylic acid (TCA) cycle flux and enhanced ATP generation via OXPHOS, underscoring a metabolic plasticity that allows cancer cells to adapt to varied microenvironmental stresses such as hypoxia and nutrient deprivation. This metabolic flexibility confers survival advantages and contributes to chemotherapy resistance, making mitochondrial bioenergetics a central hub for therapeutic exploration.
Mitochondrial dynamics proteins emerge as critical modulators of these metabolic shifts. The fission machinery, principally mediated by dynamin-related protein 1 (Drp1) and its receptor Fis1, is frequently upregulated in TNBC. Drp1 overexpression correlates with poor clinical prognosis and is implicated in enhancing Notch1-driven chemoresistance pathways. By promoting mitochondrial fragmentation, fission supports cell cycle progression, sustains cancer stemness, and facilitates metastatic dissemination.
On the other hand, mitochondrial fusion proteins, including mitofusins (MFN1/2) and optic atrophy 1 (OPA1), bolster mitochondrial networking, facilitating OXPHOS and balancing ROS levels. MFN2’s interaction with pyruvate kinase M2 (PKM2) attenuates glycolytic flux, imposing a metabolic check that counters oncogenic drive. Experimental inhibition of OPA1 diminishes tumor aggressiveness, emphasizing the nuanced role of fusion in moderating cancer phenotypes and suggesting potential targets to restrain malignancy.
Mitophagy, the selective autophagic clearance of damaged mitochondria, further intricately modulates the tumor milieu. The PINK1/Parkin pathway governs mitophagy, facilitating mitochondrial quality control and influencing ROS generation. In breast tumors deficient in BRCA1, mitophagy disruption elevates mitochondrial ROS, triggering NLRP3 inflammasome activation, a pro-inflammatory axis that enhances metastatic potential. Conversely, therapeutic promotion of mitophagy—using natural compounds like polyphyllin I and silibinin—can induce apoptosis in TNBC, revealing mitophagy’s dualistic role as both a survival mechanism and a vulnerability.
Therapeutic endeavors targeting mitochondrial dynamics have gained traction with preclinical studies illustrating that inhibiting mitochondrial fission can thwart cancer progression. Agents such as Mdivi-1, a Drp1 inhibitor, and the P110 peptide have demonstrated efficacy in reducing metastasis and restoring sensitivity to chemotherapeutic agents. Conversely, strategies that promote mitochondrial fusion, by enhancing MFN2 activity, repress glycolytic metabolism and impede tumor growth, providing a complementary avenue for intervention.
Moreover, modulating mitophagy has emerged as an innovative therapeutic modality. Compounds including warangalone and kaempferol induce excessive mitophagy, leading to mitochondrial dysfunction and cancer cell death, while others like cepharanthine counteract pro-survival mitophagy pathways. These findings underscore the therapeutic potential of finely tuning mitochondrial quality control processes to disrupt breast cancer’s resilient metabolic networks.
Despite promising progress, several challenges temper the clinical translation of mitochondrial-targeted therapies. Intratumoral heterogeneity means mitochondrial adaptations differ significantly among tumor subtypes and stages, necessitating precision medicine approaches. Furthermore, cancer cells’ metabolic plasticity often renders them adept at circumventing single-target treatments, underscoring the need for combinatorial regimens.
The realization of mitochondrial biomarkers as reliable clinical tools also remains in its infancy. Quantifying Drp1 expression or monitoring mitochondrial functional states through non-invasive technologies is critical to stratifying patients and gauging therapy responses. Adding another layer of complexity, advanced drug delivery systems, such as nanoparticle carriers engineered to selectively target tumor mitochondria, are being developed to enhance therapeutic efficacy and minimize off-target effects.
Looking forward, integrating multi-omics approaches to interrogate mitochondrial metabolism alongside immune modulation offers a promising research trajectory. Understanding the crosstalk between metabolic reprogramming and the tumor immune landscape may unveil synergistic combination treatments. Additionally, experimental therapies involving mitochondrial transplantation are being explored to restore mitochondrial function or alter metabolic dependencies within cancer cells, potentially opening transformative avenues in oncology.
In summation, mitochondrial dynamics stand at a crossroads of cellular metabolism, survival, and malignancy in breast cancer metastasis. Their regulation of fission, fusion, and mitophagy orchestrates complex adaptations that fuel tumor aggressiveness and therapy resistance. As our molecular understanding deepens, exploiting these mitochondrial processes represents a compelling strategy to dismantle the metabolic versatility that underpins treatment-refractory breast cancers. While hurdles remain, the future of mitochondrial-directed therapeutics in precision oncology shines brightly, promising renewed hope for patients battling aggressive breast cancer subtypes.
Subject of Research: Mitochondrial dynamics and metabolism in breast cancer metastasis
Article Title: Mitochondrial Dynamics in Breast Cancer Metastasis: From Metabolic Drivers to Therapeutic Targets
News Publication Date: 30-Mar-2025
Web References: DOI 10.14218/OnA.2025.00001
Image Credits: Bhuban Ruidas