In an unexpected revelation, recent research has illuminated the pervasive yet often overlooked issue of bacterial biofilms, particularly highlighting their insidious presence in everyday environments. Often recognized by the unsightly black, grey, or pink stains that stubbornly cling to bathroom tiles or kitchen sinks, these biofilms pose significant challenges in various settings, from homes to food processing facilities and even healthcare institutions. Traditional cleaning methods, including intense scrubbing and the use of disinfectants, frequently fall short in eliminating these resilient structures. This raises critical questions about our understanding and management of bacterial biofilms, leading to an innovative study published in the esteemed Chemical Engineering Journal.
At the forefront of this study is Hyunjoon Kong, a professor of chemical and biomolecular engineering, who articulates the widespread implications of biofilms. “Biofilms are everywhere, from bathrooms to food factories,” Kong asserts. Their role is especially concerning in hospital settings, where they can contribute to cross-contamination among patients and settle on medical devices such as tooth and bone implants. This highlights the dual challenge of addressing public hygiene and patient safety, marking a significant point of concern for researchers and public health officials alike.
Bacterial biofilms, contrary to popular belief, are not merely collections of free-floating bacteria. Instead, they constitute complex communities enveloped within a formidable external 3D matrix that provides not only structural support but also a defensive barrier against external attacks, including chemical disinfectants and antibiotics. This matrix facilitates a coordinated defense among bacterial cells, thereby enhancing their survival rate compared to isolated cells. Unfortunately, the existing treatments often target only the outer layers of these biofilms, leaving underlying bacteria protected and capable of resurgence.
The new study explores the nuanced interactions between bacterial cells and their matrix, aiming to dismantle these protective barriers more effectively. The research team investigated the performance of chemical disinfectants on Pseudomonas aeruginosa biofilms, a notorious biofilm-forming pathogen. By employing various treatments such as hydrogen peroxide (H₂O₂) and a mixture of H₂O₂ with peroxyacetic acid (PAA), the researchers assessed their effectiveness in eradicating the biofilm. Despite initial success in killing the majority of the bacteria, the findings revealed a troubling pattern: biofilm regrowth was evident within 24 hours after treatment.
This study underscores a significant gap in our current disinfection methods, as the chemical agents employed even at lethal concentrations struggled to breach the protective matrix. “We thought that maybe these chemicals kill the cells but don’t effectively damage the matrix,” Kong reflects. This raised the possibility that surviving cells could exploit remnants of the matrix to reestablish the biofilm.
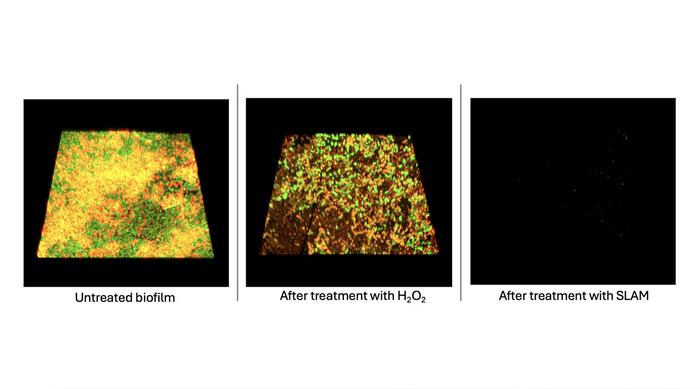
In a groundbreaking approach, Kong and his team integrated advanced imaging techniques with immunostaining to develop intricate 3D renderings of the biofilms. By analyzing these renderings, they could critically assess how the extracellular polymeric substances (EPS) interacted with the bacterial cells and identified that while the chemical disinfectants managed to remove over 50% of the biofilm, a substantial residual matrix remained. The persistence of the matrix provided a conducive environment for the surviving bacteria, thus fuelling regrowth.
Continuing their investigation, the researchers sought to harness self-locomotive antibacterial microbubblers, known as SLAM, previously engineered by Kong’s team. These microbubblers, when activated using H₂O₂, produce oxygen bubbles that infiltrate biofilm structures, leading to mechanical disruption. Through a novel two-step process, they hypothesized that SLAM could first destabilize the interactions between the cells and their matrix, followed by a targeted application of H₂O₂ and PAA to eliminate residual bacteria.
Remarkably, testing this combined approach yielded promising results. After sequential treatment with SLAM followed by H₂O₂ and PAA, the researchers reported a staggering 95% removal of both the matrix and bacterial cells. More importantly, 24 hours post-treatment, there was no observable regrowth of Pseudomonas aeruginosa, and the biofilm’s resurgence was hindered for over two months. These findings not only provide a potential pathway to combat biofilm-related infections but also stress the importance of understanding the underlying mechanics of biofilm formation and regrowth.
Transitioning this innovative technology from a laboratory setting to practical application is a crucial next step. Kong’s team is dedicated to adapting their findings for real-world contexts, especially in the field of dental implant disinfection, aiming to ensure that clinically used devices are free from harmful bacterial colonization. Their vision also extends to scaling manufacturing techniques for SLAM particles, making them accessible for wider utilization in various industries.
Despite these promising advancements, Kong acknowledges that further research is essential. A comprehensive understanding of biofilm behavior, particularly regarding the chemical and mechanical properties of the protective matrices, remains imperative. “If we gain more information about how biofilms resist antibiotics, we can better develop our strategies to combat these infections,” he expresses, emphasizing the intricate complexity of these microscopic entities.
In conclusion, the fight against bacterial biofilms is not only a matter of hygiene but a critical public health challenge that demands our attention. This study exemplifies how novel approaches and enhanced understanding can lead to revolutionary disinfection methods, potentially transforming not only the way we manage cleanliness in our surroundings but also how we safeguard patient health in clinical settings.
Subject of Research: Disruption of bacterial biofilms using SLAM particles.
Article Title: Biofilm comes back: Controlling regrowth by mitigating the cell-matrix interaction
News Publication Date: 25-Feb-2025
Web References: Chemical Engineering Journal DOI
References: Published in Chemical Engineering Journal, 25-Feb-2025
Image Credits: Joon Kong, Yu-Heng Deng, Joohun Lee
Keywords
Biofilms, Pseudomonas, biofilm formation, biofilm infections, microparticles.