In a groundbreaking new study poised to redefine our understanding of gastric cancer, researchers have identified a previously uncharted molecule—tRF-29-79MP9P9NH525—that acts both as a crucial biomarker and a potent tumor suppressor. This discovery pivots around a complex regulatory axis involving the KIF14/AKT signaling pathway, offering a promising avenue for novel therapeutic strategies against one of the most insidious and prevalent malignancies worldwide. Published in the prestigious journal Cell Death Discovery, this work unravels the intricate molecular interplay that could soon translate into clinical breakthroughs, delivering hope for millions affected by gastric cancer.
Gastric cancer remains one of the leading causes of cancer-related mortality globally, largely due to late diagnosis and limited effective treatments. The molecular heterogeneity and elusive pathogenesis of this disease have long challenged scientists striving to decode its underlying biology. This study by Ge, J., Dai, J., Ji, H., and colleagues introduces tRF-29-79MP9P9NH525, a newly characterized tRNA-derived fragment (tRF), which emerges as a pivotal player in suppressing tumor progression. This molecule’s dual role as both a biomarker for early detection and a modulator of tumor growth marks a significant advancement in gastric cancer research.
Transfer RNA-derived fragments, or tRFs, are an expanding class of small non-coding RNAs initially considered incidental degradation products. However, recent scientific scrutiny has illuminated their far-reaching functional versatility, particularly in cancer biology. The identified tRF-29-79MP9P9NH525 exhibits a sophisticated regulatory capacity by interfacing with the KIF14/AKT pathway, a critical signaling route known for orchestrating cell proliferation, survival, and metabolism. These molecular relationships shed light on a previously unappreciated tumor-suppressive mechanism governed by tRFs.
The KIF14/AKT pathway itself occupies a central node in oncogenic signaling networks. KIF14, a member of the kinesin family involved in intracellular transport and mitotic processes, frequently shows aberrant expression in various cancers, promoting tumor aggressiveness. AKT, also recognized as protein kinase B, orchestrates multiple downstream effectors that foster proliferation and inhibit apoptotic processes. Modulating these pathways has been a focal point of targeted therapy development, but the precise upstream regulators have remained elusive—until now.
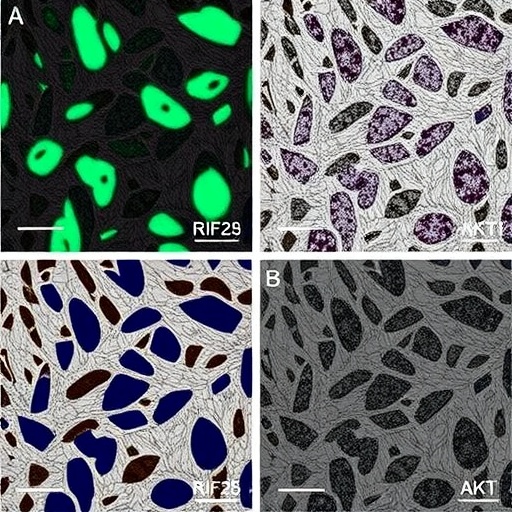
By deploying an integrative approach combining high-throughput RNA sequencing, molecular biology assays, and functional in vitro and in vivo experiments, the researchers meticulously characterized tRF-29-79MP9P9NH525’s expression profile and mechanistic role. Their data reveal that this tRF is significantly downregulated in gastric cancer tissues compared to normal counterparts, correlating inversely with tumor stage and patient prognosis. This dichotomous expression profile underscores its viability as a prognostic biomarker, enabling earlier and more precise detection modalities.
Functionally, overexpression of tRF-29-79MP9P9NH525 in gastric cancer cell lines triggered a profound repression of proliferation rates and invasive capabilities. The authors demonstrate that this molecule achieves tumor suppression by attenuating the activity of KIF14, resulting in downstream inhibition of the AKT signaling cascade. Consequent reductions in AKT phosphorylation diminish the survival signaling pathways that normally shield tumor cells from apoptosis, thereby sensitizing them to programmed cell death mechanisms.
Beyond cellular assays, the in vivo models reinforce these findings, where animal subjects receiving tRF-29-79MP9P9NH525 mimetics exhibited markedly reduced tumor growth and metastatic spread compared to controls. These compelling results not only validate the molecular pathway elucidated but also signify translational potential. Therapeutic strategies harnessing synthetic analogs or delivery systems to restore or amplify tRF-29-79MP9P9NH525 expression are on the horizon, promising to enhance existing gastric cancer treatments or provide standalone options.
Perhaps more intriguing is the implication of tRF biology in the broader context of RNA therapeutics. Unlike traditional protein-targeted drugs, tRFs, as small RNA molecules, afford unique advantages including high specificity, low immunogenicity, and modifiable stability. This elevates them to a new class of biomolecules with the power to modulate complex intracellular signaling networks with precision. The current study situates tRF-29-79MP9P9NH525 at the vanguard of this emerging therapeutic frontier.
Nonetheless, challenges remain before clinical application can become a reality. Delivery modalities for RNA-based therapies, potential off-target effects, and long-term safety profiles warrant meticulous examination. Additionally, the heterogeneity of gastric cancer across patient populations necessitates validation in diverse cohorts to confirm the universality of tRF-29-79MP9P9NH525-mediated regulatory mechanisms. The authors advocate for further exploration into the molecular interactions and possible co-factors influencing tRF function to refine therapeutic targeting.
Moreover, the research opens exciting avenues for biomarker development beyond gastric cancer. Given the conserved nature of tRFs and the ubiquitous presence of KIF14/AKT signaling dysregulation in multiple cancer types, analogous mechanisms might be operative elsewhere. Screening for aberrations in tRF expression could revolutionize early detection paradigms across oncology, facilitating personalized medicine approaches and real-time monitoring of treatment efficacy.
The study also underscores the profound impact of integrating computational analytics with experimental biology. Advanced bioinformatics tools deciphered intricate RNA profiles from extensive datasets, enabling the pinpointing of tRF-29-79MP9P9NH525 amidst a sea of candidates. This multidimensional investigative strategy exemplifies the future of biomedical discovery, where big data and molecular experimentation converge to unlock new biological insights.
From a clinical perspective, the dual functionality of tRF-29-79MP9P9NH525 as both biomarker and tumor suppressor streamlines its potential utility. Non-invasive assays such as liquid biopsies could measure circulating levels of this tRF, affording clinicians a dynamic window into tumor status and therapeutic response. Simultaneously, augmenting its tumor suppressor function might deliver therapeutic benefit through direct modulation of oncogenic pathways.
This research challenges existing dogmas regarding non-coding RNAs, positioning tRFs as critical regulators of cancer biology rather than mere transcriptional noise. The complex interplay between tRF-29-79MP9P9NH525 and the KIF14/AKT axis exemplifies an elegant regulatory network that restrains malignancy, offering hope that targeting these fine molecular levers may unleash powerful anti-cancer effects with minimal collateral damage.
In summation, the elucidation of tRF-29-79MP9P9NH525’s role in gastric cancer signifies a paradigm shift, marrying fundamental molecular biology with translational medicine to tackle one of oncology’s deadliest diseases. As the scientific community continues to decode the multifaceted roles of tRFs and their intersecting pathways, this discovery will likely catalyze further innovations, bringing us closer to effective, personalized therapies that can dramatically improve patient outcomes in gastric cancer and beyond.
Subject of Research: Gastric cancer; tumor-suppressive tRNA-derived fragment; KIF14/AKT signaling pathway
Article Title: Identification of tRF-29-79MP9P9NH525 as a biomarker and tumor suppressor of gastric cancer via regulating KIF14/AKT pathway
Article References:
Ge, J., Dai, J., Ji, H. et al. Identification of tRF-29-79MP9P9NH525 as a biomarker and tumor suppressor of gastric cancer via regulating KIF14/AKT pathway. Cell Death Discov. 11, 238 (2025). https://doi.org/10.1038/s41420-025-02514-9
Image Credits: AI Generated