Hydrogenation—the chemical reaction of hydrogen gas with another compound—is fundamental in industries such as food, pharmaceuticals, materials, and petrochemicals. Traditionally, noble metals like palladium and rhodium serve as catalysts in these reactions. However, these materials are scarce and expensive, and their mining is plagued by environmental concerns. Moreover, they demand highly controlled and energy-intensive conditions to function effectively.

Credit: Tokyo Institute of Technology
Hydrogenation—the chemical reaction of hydrogen gas with another compound—is fundamental in industries such as food, pharmaceuticals, materials, and petrochemicals. Traditionally, noble metals like palladium and rhodium serve as catalysts in these reactions. However, these materials are scarce and expensive, and their mining is plagued by environmental concerns. Moreover, they demand highly controlled and energy-intensive conditions to function effectively.
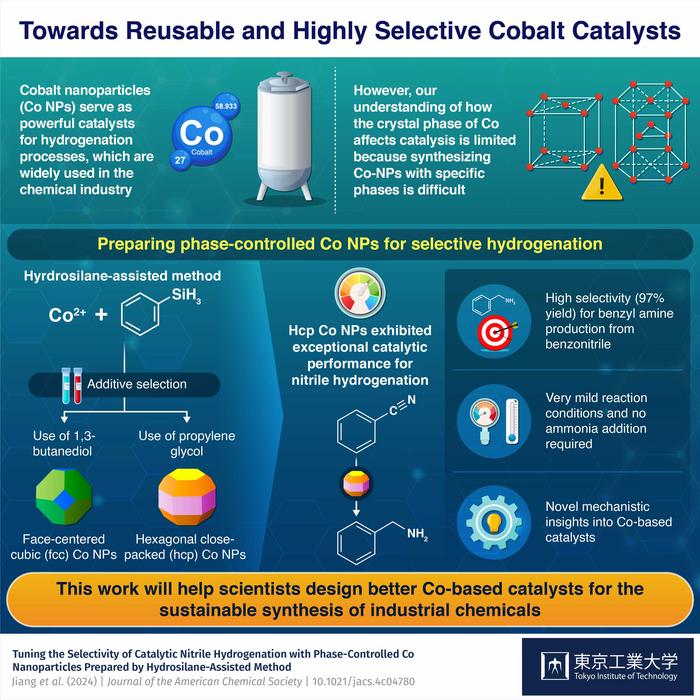
In recent years, cobalt has emerged as a promising alternative to noble metal catalysts for hydrogenation. Cobalt nanoparticles (Co NPs) can catalyze hydrogenation reactions efficiently, requiring lower temperatures and pressures than noble metal catalysts. Despite the theoretical significance of the crystal phase of Co NPs in their catalytic performance, studying this has been challenging due to the lack of methods to produce Co NPs with a specific crystal phase of similar size.
Fortunately, a research team from Tokyo Institute of Technology and Osaka Metropolitan University, has found a solution to this issue. As reported in their latest study, which was published in the Journal of the American Chemical Society, they devised a new method to selectively produce Co NPs with two very distinct crystal phases in a convenient and consistent way.
“We previously reported that nickel NPs can be readily synthesized by the reduction of nickel complexes in the presence of hydrosilanes through a technique we dubbed the hydrosilane-assisted method,” explains Professor Michikazu Hara, who led the study, “We hypothesized hydrosilanes would act not only as reducing agents for Co cations, but also as ligands on the metal complexes to control the growth of metal particles. Therefore, we attempted to synthesize Co NPs with a controlled crystal phase via the simple addition of appropriate coordination compounds and specific diol solvents.”
Through careful testing, the research team found their predictions were right, and the hydrosilane-assisted method could reliably produce two types of Co NPs depending on the reaction conditions: face-centered cubic (fcc) Co NPs and hexagonal close-packed (hcp) Co NPs. They then ran several hydrogenation experiments to explore the differences in catalytic performance between both types of Co NPs.
Interestingly, they observed that hcp-Co NPs were far superior to fcc-Co NPs. In the hydrogenation of benzonitrile into benzyl amine, hcp-Co NPs achieved a much higher selectivity, exhibiting a yield of 97% compared to fcc-Co NPs’ 80%. Moreover, hydrogenation using hcp-Co NPs required a pressure of roughly half that of fcc-Co NPs, rendering the entire process more energy-efficient. This notable feat is not limited to the production of benzyl amine only, as Hara remarks: “Worth noting, the proposed Co NP-based catalytic system displayed compatibility with a diverse range of nitriles and carbonyl compounds.”
Considering that cobalt is abundant, and the proposed Co NP-base catalyst is reusable and does not require harmful gases such as ammonia, this work could serve as a stepping stone towards more sustainable and cost-effective hydrogenation technologies. In turn, this will hopefully lower the price and environmental impact of many industrial commodities, drugs, and even food products, leading to an overall better future for humanity in multiple ways.
###
About Tokyo Institute of Technology
Tokyo Tech stands at the forefront of research and higher education as the leading university for science and technology in Japan. Tokyo Tech researchers excel in fields ranging from materials science to biology, computer science, and physics. Founded in 1881, Tokyo Tech hosts over 10,000 undergraduate and graduate students per year, who develop into scientific leaders and some of the most sought-after engineers in industry. Embodying the Japanese philosophy of “monotsukuri,” meaning “technical ingenuity and innovation,” the Tokyo Tech community strives to contribute to society through high-impact research.
Institute of Science Tokyo (Science Tokyo) will be established on October 1, 2024, following the merger between Tokyo Medical and Dental University (TMDU) and Tokyo Institute of Technology (Tokyo Tech), with the mission of “Advancing science and human wellbeing to create value for and with society.”
Journal
Journal of the American Chemical Society
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Tuning the Selectivity of Catalytic Nitrile Hydrogenation with Phase-Controlled Co Nanoparticles Prepared by Hydrosilane-Assisted Method
Article Publication Date
18-Jul-2024