A groundbreaking multinational study has shed new light on immune-mediated adverse events (irAEs) associated with the combination therapy of atezolizumab and bevacizumab in patients with unresectable hepatocellular carcinoma (HCC) across Latin America. This comprehensive real-world investigation, recently published in the esteemed journal Oncotarget, stands as one of the first to explore how this patient population responds to such cutting-edge immunotherapy outside controlled clinical trial environments. The findings offer pivotal insights that could reshape clinical management strategies for HCC, a notoriously aggressive form of liver cancer with limited therapeutic options.
Hepatocellular carcinoma remains a global health challenge due to its high mortality, often stemming from late diagnosis and the limited efficacy of conventional treatments in advanced stages. The advent of immunotherapy—specifically immune checkpoint inhibitors like atezolizumab, an anti-PD-L1 antibody, combined with bevacizumab, an anti-VEGF monoclonal antibody—has revolutionized treatment paradigms. These agents reinvigorate the patient’s immune system to target tumor cells, yet this immune activation can provoke adverse systemic effects collectively termed immune-related adverse events (irAEs). These adverse responses, while potentially severe, are incompletely characterized in Latin American populations, particularly within the multifaceted clinical contexts typical of everyday medical practice.
To address this knowledge gap, researchers conducted a multicentric retrospective cohort study encompassing 99 patients with advanced unresectable HCC treated between 2019 and 2024 across Argentina, Brazil, Chile, and Colombia. These patients received the atezolizumab and bevacizumab regimen under routine clinical care, providing invaluable real-world data. The median treatment duration was approximately six months, reflecting the typical clinical course for this demographic. Crucially, the investigators meticulously documented incidence rates, severity, and organ-specific manifestations of irAEs, as well as their relationship to overall survival outcomes.
Intriguingly, only 18% of the cohort experienced immune-mediated toxicities, a noticeably lower frequency compared to prior randomized clinical trials where irAE rates often exceed 30%. The predominant organ systems involved were the liver, manifesting as immune-mediated hepatitis, and the thyroid gland, causing thyroiditis. Most irAEs were graded as mild to moderate, corresponding to Common Terminology Criteria for Adverse Events (CTCAE) grades 1 or 2, and resolved rapidly within approximately 30 days following appropriate clinical intervention. Steroid therapy was required in just eight cases for immune suppression, underscoring that most irAEs were manageable without aggressive immunomodulation.
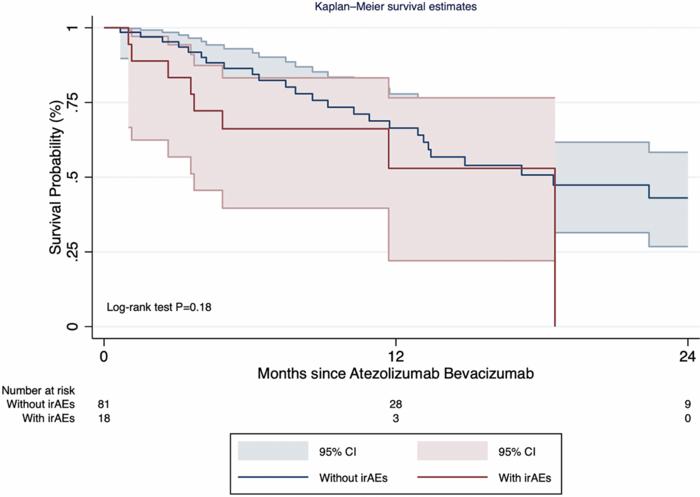
From a survival perspective, the study’s Kaplan-Meier analyses demonstrated no statistically significant difference in median overall survival between patients who developed irAEs and those who did not; both groups exhibited a median survival of 18.5 months. This finding challenges prior hypotheses suggesting that immune toxicities correlate with enhanced antitumor efficacy. Instead, it supports the clinical notion that irAEs, while necessitating vigilance, do not inherently negate the therapeutic benefits of atezolizumab and bevacizumab. Therefore, prompt recognition and tailored management of irAEs remain key to optimizing patient outcomes.
A particularly compelling aspect of this research was the identification of elevated baseline alpha-fetoprotein (AFP) levels—specifically values exceeding 400 ng/mL—as a significant predictor for the development of irAEs. AFP, a well-established biomarker linked to tumor burden and aggressiveness in HCC, may therefore serve as a valuable tool to stratify patients’ risk for immune toxicity. This predictive association empowers clinicians to implement intensified monitoring protocols or preemptive strategies in high-risk patients, potentially improving the safety profiles of immunotherapeutic regimens.
The study also highlights the distinct nature of real-world evidence compared to stringent clinical trial data. Trial participants often undergo rigorous monitoring and follow-up, with detailed recording of adverse events, which might inflate irAE incidence statistics relative to everyday clinical settings. Variability in patient demographics, comorbidities, and healthcare infrastructure across Latin America further contributes to heterogeneity in treatment responses and side-effect profiles. These factors underscore the importance of region-specific data to guide practical clinical decision-making and resource allocation.
Moreover, the researchers emphasize the dynamic interplay between immunotherapy and underlying liver disease. Many enrolled patients had cirrhosis or other chronic hepatic conditions that can complicate the immunological landscape, potentially masking or mimicking irAEs. This complexity necessitates nuanced clinical judgment to differentiate adverse events from disease progression or decompensation. The study’s findings advocate for multidisciplinary collaborations involving oncologists, hepatologists, and immunologists to optimize patient care.
From a mechanistic standpoint, the study reaffirms the dualistic nature of immunotherapy in cancer treatment. While agents like atezolizumab unleash cytotoxic T-cell responses to eradicate tumor cells, they can inadvertently disrupt immune tolerance, precipitating autoimmune-like toxicities. Bevacizumab’s anti-angiogenic effects add another layer, modulating tumor vasculature and potentially influencing immune cell trafficking. Understanding these interactions at a molecular level remains a crucial research frontier, with implications for designing next-generation therapies that maximize antitumor activity while minimizing collateral damage.
Importantly, the study contributes to the broader discourse on health disparities and the generalizability of clinical trial findings. Latin American countries often face challenges such as limited access to advanced therapeutics, variations in healthcare delivery, and underrepresentation in global studies. By focusing on this cohort, the authors provide data that acknowledges regional specificities, fostering equitable improvements in cancer care. This approach aligns with global initiatives promoting inclusivity and diversity in oncology research.
In conclusion, this landmark study elucidates the incidence, clinical characteristics, and prognostic implications of immune-mediated adverse events in Latin American patients with advanced hepatocellular carcinoma treated with atezolizumab and bevacizumab. The findings underscore that while irAEs are relatively uncommon and generally manageable, their occurrence does not adversely impact overall survival. Elevated AFP emerges as a promising biomarker to identify individuals at higher risk for toxicity. These real-world insights reinforce the necessity for vigilant, individualized management to harness the full potential of immunotherapy in HCC, ultimately striving toward improved patient outcomes and quality of life.
Subject of Research: People
Article Title: Immune-mediated adverse events following atezolizumab and bevacizumab in a multinational Latin American cohort of unresectable hepatocellular carcinoma
News Publication Date: 19-May-2025
Web References:
- Oncotarget Volume 16: https://www.oncotarget.com/archive/v16/
- DOI link: http://dx.doi.org/10.18632/oncotarget.28721
Image Credits: Copyright: © 2025 da Fonseca et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
Keywords: liver cancer; immunotherapy; adverse events; immunology; real-world