Toronto, Ontario (Embargoed until 9:30 a.m. EDT, Sunday, June 9, 2024)—New data on prolonged prostate-specific antigen (PSA) progression and adverse events grouped by safety topic of interest from the PSMAfore study underscore the statistically significant and clinically meaningful radiographic progression-free survival (rPFS) benefit of [177Lu]Lu-PSMA-617 radioligand therapy for patients with taxane-naive metastatic castration-resistant prostate cancer (mCRPC). This study was presented at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2024 Annual Meeting and was honored as the meeting’s Abstract of the Year.
Each year, SNMMI chooses an abstract that best exemplifies the most promising advances in the field of nuclear medicine and molecular imaging. This year, the SNMMI Henry N. Wagner, Jr., Abstract of the Year was chosen from more than 1,500 abstracts submitted to the meeting and voted on by reviewers and the society leadership.

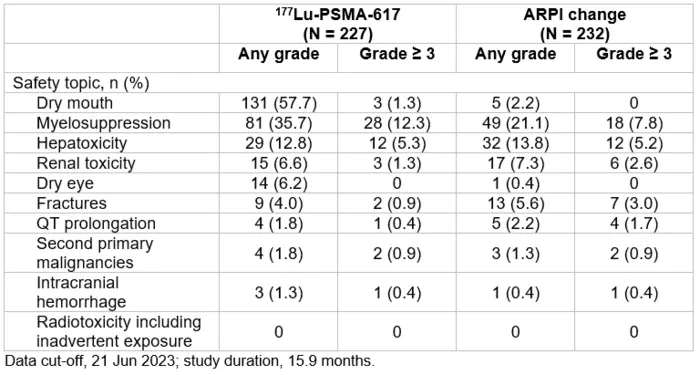
Credit: Table created by Ken Herrmann, Oliver Sartor, et al.
Toronto, Ontario (Embargoed until 9:30 a.m. EDT, Sunday, June 9, 2024)—New data on prolonged prostate-specific antigen (PSA) progression and adverse events grouped by safety topic of interest from the PSMAfore study underscore the statistically significant and clinically meaningful radiographic progression-free survival (rPFS) benefit of [177Lu]Lu-PSMA-617 radioligand therapy for patients with taxane-naive metastatic castration-resistant prostate cancer (mCRPC). This study was presented at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2024 Annual Meeting and was honored as the meeting’s Abstract of the Year.
Each year, SNMMI chooses an abstract that best exemplifies the most promising advances in the field of nuclear medicine and molecular imaging. This year, the SNMMI Henry N. Wagner, Jr., Abstract of the Year was chosen from more than 1,500 abstracts submitted to the meeting and voted on by reviewers and the society leadership.
“Findings from the pivotal Phase 3 VISION trial led to the U.S. Food and Drug Administration (FDA) approval of [177Lu]Lu-PSMA-617 for PSMA-positive mCRPC patients previously treated with both an androgen receptor pathway inhibitor (ARPI) and taxane-based chemotherapy,” said Ken Herrmann, MD, chair of the Department of Nuclear Medicine at University Hospital Essen in Essen, Germany. “The current PSMAfore study aims to assess the benefit of [177Lu]Lu-PSMA-617 in patients with mCRPC who have never received taxane-based chemotherapy with the hope of making this promising therapy available to more patients earlier in the course of their treatment journey.”
The study included 468 taxane-naive mCRPC patients who were prostate-specific membrane antigen (PSMA)-positive on [68Ga]Ga-PSMA-11 PET/CT imaging and candidates for an ARPI change after one progression on prior ARPI therapy. Patients were randomly assigned to one of two treatments: [177Lu]Lu-PSMA-617 or a change in ARPI (to abiraterone or enzalutamide). Researchers assessed rPFS (primary endpoint) and overall survival (key secondary endpoint), as well as PSA decline of 50 percent or more from baseline (PSA50), health-related quality of life, and safety. In the PSMAfore study, [177Lu]Lu-PSMA-617 was well tolerated with a manageable safety profile consistent with the established safety profile and good tolerability observed in the VISION trial that assessed patients with mCRPC treated with ARPI and taxane-based chemotherapy. Time to PSA progression and objective response rate were also evaluated. Researchers analyzed data for patients in both the [177Lu]Lu-PSMA-617 and ARPI change groups at 7.3 months (primary analysis) and again at 15.9 months (second interim analysis).
At primary analysis, the median rPFS was 9.30 months for the [177Lu]Lu-PSMA-617 group and 5.55 months for ARPI change group (HR 0.41, 95% CI 0.29-0.56); results were similar at the second interim analysis. Data collected at the second interim analysis also show that for [177Lu]Lu-PSMA-617 versus ARPI change, median time to worsening in FACT-P total score was longer (7.46 months versus 4.27 months), objective response rate was higher (50.7 percent versus 14.9 percent), PSA50 response was higher (57.6 percent versus 20.4 percent), and median time to PSA progression was longer (10.55 months versus 4.24 months). Incidence of grade >3 adverse events in the second interim analysis were fewer for the [177Lu]Lu-PSMA-617 group than for the ARPI change group. In the [177Lu]Lu-PSMA-617 group, the most common groups of any-grade treatment-emergent adverse events according to safety topics of interest were dry mouth and myelosuppression – a condition in which bone marrow activity is decreased, resulting in fewer red blood cells (anemia), white blood cells (thrombocytopenia), and platelets (neutropenia).
Updated overall survival results from the third interim analysis will be presented in an oral presentation this afternoon. Adding to the extensive knowledge on the efficacy and tolerability of [177Lu]Lu-PSMA-617, these latest findings highlight the potential of [177Lu]Lu-PSMA-617 as a treatment option for patients in the earlier lines of advanced prostate cancer.
“Patients with advanced prostate cancer face an unmet need for treatments that offer clinically meaningful benefits while minimizing impact of severe adverse events. Results from the PSMAfore study suggest that [177Lu]Lu-PSMA-617 may have the potential to change the treatment paradigm for patients with advanced prostate cancer in the pre-taxane setting,” noted Oliver Sartor, MD, Director of Radiopharmaceutical Trials at the Mayo Clinic in Rochester, Minnesota and presenter of today’s oral abstract.
Plans are underway to submit an application to the FDA for a label expansion for [177Lu]Lu-PSMA-617 in the pre-taxane setting for the second half of 2024, according to Novartis, the trial sponsor. If approved, these data will be incorporated into the updated product prescribing information, providing earlier access to this therapy.
Abstract 241064. “Phase 3 trial of [177Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (PSMAfore),” Ken Herrmann, Oliver Sartor, Daniel Castellano, Johann de Bono, Neal Shore, Kim Chi, Mike Crosby, Josep Piulats, Aude Flechon, Xiao Wei, Hakim Mahammedi, Guilhem Roubaud, Hana Študentová, Samson Ghebremariam, Euloge Kpamegan, Teri Kreisl, Neda Delgoshaie, Katja Lehnhoff, Michael Morris, Karim Fizazi, Department of Nuclear Medicine, University Hospital Essen, Essen, Germany.
###
All 2024 SNMMI Annual Meeting abstracts can be found online.
About the Society of Nuclear Medicine and Molecular Imaging
The Society of Nuclear Medicine and Molecular Imaging (SNMMI) is an international scientific and medical organization dedicated to advancing nuclear medicine and molecular imaging—vital elements of precision medicine that allow diagnosis and treatment to be tailored to individual patients in order to achieve the best possible outcomes.
SNMMI’s members set the standard for molecular imaging and nuclear medicine practice by creating guidelines, sharing information through journals and meetings and leading advocacy on key issues that affect molecular imaging and therapy research and practice. For more information, visit www.snmmi.org.
Journal
Journal of Nuclear Medicine
Article Title
Phase 3 trial of [177Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (PSMAfore)
Article Publication Date
9-Jun-2024