In the relentless battle against glioblastoma, one of the most aggressive and treatment-resistant brain cancers, scientists at The Herbert Wertheim UF Scripps Institute for Biomedical Innovation & Technology have unveiled a groundbreaking therapeutic strategy that could redefine the future of oncology. Their pioneering work centers on a novel compound, MT-125, which has demonstrated unprecedented efficacy in sensitizing glioblastoma tumors to radiation and chemotherapy, thereby halting their invasive progression in preclinical models. This new approach, detailed in a recent publication in Cell, leverages the targeting of cellular “motors” — nanoscale myosin proteins essential for cancer cell survival and motility — offering a potential lifeline to thousands of patients who currently face dismal prognoses.
Glioblastoma is notorious for its aggressive nature and poor patient survival, with standard-of-care treatments rarely extending life beyond 14 to 16 months post-diagnosis. The heterogeneity of this malignancy, compounded by molecular subtypes resistant to existing chemotherapy agents, underscores the urgent need for innovative treatment modalities. Recognizing this, the research team embarked on a mission to dissect the molecular underpinnings of glioblastoma’s resilience. They identified the myosin motor proteins—fundamental components that convert chemical energy into mechanical forces within cells—as key facilitators in tumor expansion and resistance mechanisms.
Myosin motors operate within a cellular environment much like miniature machines, orchestrating diverse processes such as motility, shape change, and intracellular transport. Their critical involvement in muscle cells is well-known, but their role in pathological states, including cancer progression, has remained largely unexploited due to the scarcity of selective pharmacological inhibitors. This gap presented both a challenge and an opportunity. By engineering a suite of small-molecule inhibitors capable of selectively incapacitating myosin motors involved in glioblastoma pathology, the team aimed to disrupt the cancer’s cellular machinery at a fundamental level.
The medicinal chemistry efforts, helmed by Dr. Theodore Kamenecka in collaboration with structural biologist Dr. Patrick Griffin, culminated in the synthesis of MT-125, a molecule specifically designed to inhibit non-muscle myosin II (NMII) functions within malignant cells. Early experimental models revealed that MT-125 impedes the contractile forces that cancer cells deploy to invade adjacent brain tissue, effectively "locking" them in place. This biophysical blockade stifles the tumor’s notorious ability to infiltrate and colonize new niches within the brain, which is a primary factor contributing to patient mortality.
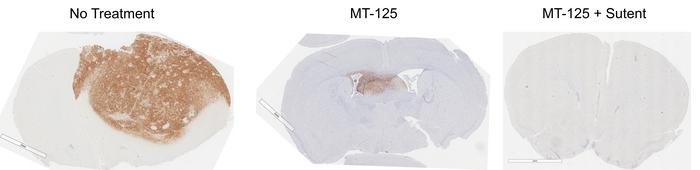
A hallmark discovery in the research was MT-125’s ability to convert glioblastoma cells from radiation-resistant phenotypes into radiation-sensitive ones. Treated cells exhibited multinucleation—a condition where cells fail to undergo proper division and become marked for programmed cell death. This mechanistic insight was corroborated through murine models, where MT-125, both as a monotherapy and in combination with the kinase inhibitor sunitinib, elicited dramatic tumor regressions. These findings suggest a synergistic augmentation of existing chemotherapeutic regimens, opening avenues for combinatorial therapies with enhanced potency.
Despite the promising outcomes, the researchers caution against premature extrapolation to human clinical success. The biological divergence between murine models and human patients necessitates cautious optimism, with comprehensive toxicity profiling and dosing strategies integral to future studies. Notably, MT-125 displays preferential toxicity towards cancer cells over healthy tissue and possesses a pharmacokinetic profile suitable for pulsed administration, which may mitigate adverse effects commonly associated with chemotherapy.
The therapeutic significance of targeting molecular motors extends beyond glioblastoma. The science behind MT-125 opens a new frontier where disabling the mechanical underpinnings of malignant cells can be harnessed across a spectrum of cancers, potentially transforming treatment paradigms. Such a strategy veers away from traditional methods that primarily target genetic signals, focusing instead on the biophysical mechanisms essential to tumor progression.
In parallel with their oncology research, the team is advancing a related compound, MT-110, which holds promise in addressing methamphetamine use disorder by modulating myosin motor-driven neuronal pathways associated with drug craving. This illustrates the broad therapeutic potential of myosin motor inhibitors, resonating beyond cancer treatment to neurological and psychiatric diseases.
The pathway to bringing MT-125 from bench to bedside is well underway. The compound has been licensed to Myosin Therapeutics, a biotechnology startup founded by the principal investigators. With FDA approval granting clearance to initiate clinical trials, the team anticipates enrolling glioblastoma patients within the year. Substantial funding from the National Institutes of Health and dedicated glioblastoma research endowments supports this ambitious effort, laying the foundation for translational success.
Clinical trials will critically evaluate safety, dosing regimens, and efficacy in the complex and heterogeneous landscape of human glioblastoma. If successful, MT-125 could herald a new era where intractable brain tumors are rendered vulnerable to existing therapies, dramatically improving patient outcomes that have remained stagnant for decades.
This landmark research embodies the impact of interdisciplinary collaboration—melding medicinal chemistry, structural biology, neuro-oncology, and clinical expertise—to tackle one of the most formidable challenges in cancer treatment. By reimagining glioblastoma therapy through the lens of cellular mechanics, the scientists have illuminated a transformative therapeutic axis poised to advance the future of oncology.
Subject of Research: Animals
Article Title: Scientists wipe out aggressive brain cancer tumors by targeting cellular ‘motors’
News Publication Date: 1-Jul-2025
Web References:
- Research article in Cell: https://www.cell.com/cell/fulltext/S0092-8674(25)00569-0
- DOI link: http://dx.doi.org/10.1016/j.cell.2025.06.006
Image Credits: Image courtesy Steven Rosenfeld, M.D., Ph.D., and Courtney Miller, Ph.D.
Keywords: Glioblastomas, Brain cancer, Cancer