Age-related macular degeneration (AMD) serves as the leading cause of vision loss in individuals aged 65 and older, manifesting through notable abnormalities within the macula, which can lead to significant visual impairments and distorted perceptions of objects. This degenerative condition predominantly affects older adults, with dry AMD constituting approximately 90% of all cases. While dry AMD typically results in mild vision issues, a concerning statistic indicates that nearly 30% of those affected may progress to the more severe form known as wet AMD within a decade. Currently, the FDA has approved two injectable therapies for treating dry AMD, but these approaches face limitations, particularly in terms of complications associated with intravitreal injections, alongside their modest effectiveness in restoring sight.
In light of these challenges, a research team spearheaded by Dr. Moon-Hyeong Seo at the Natural Product Drug Development Center of the Korea Institute of Science and Technology (KIST) has made significant strides in developing a novel therapeutic agent for dry AMD. This groundbreaking new treatment can be conveniently administered via eye drops, addressing a long-standing preference for non-invasive drug delivery systems within the ophthalmic market. Despite the high demand for eye drop formulations, targeting the retina—a region situated in the posterior segment of the eye—has posed considerable challenges for researchers.
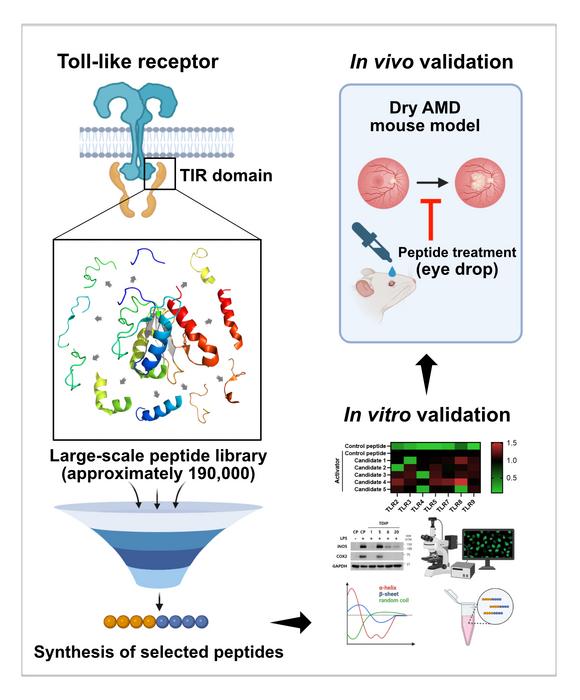
The research team adopted a strategic focus on the inflammatory signaling pathways mediated by Toll-like receptors (TLRs), proteins integral to the progression of AMD. By meticulously extracting peptide sequences from tens of thousands of proteins that share structural similarities with natural TLR signaling molecules, they established an expansive library comprising over 190,000 peptide drug candidates. Leveraging cutting-edge technology, the researchers expedited the screening process, successfully identifying numerous candidate peptides capable of inhibiting undesirable interactions between TLR signaling proteins, a crucial step in mitigating disease progression.
To ascertain the therapeutic potential of the identified peptides, the team conducted rigorous testing by administering these eye drops to mice with induced dry AMD. The results were promising: the treated mice displayed substantial retinal cell protection and a marked reduction in retinal degeneration, outcomes that paralleled those observed in normal mice. This pivotal finding suggests that peptide-based eye drops may represent a viable alternative to the conventional injectable therapies currently employed to treat dry AMD.
Transitioning to an eye drop formulation not only enhances the convenience of treatment for patients but also promotes better adherence to therapy, significantly reducing complications and costs associated with routine invasive procedures. The non-invasive nature of the therapy presents a safe avenue for patients, offering a new horizon for managing AMD and other related ophthalmic conditions. This innovative approach stands to revolutionize treatment accessibility and overall patient experience, paving the way for broader applications in the field of ophthalmology.
Dr. Seo emphasized the commitment of the KIST Natural Product Drug Development Center, which was established in September, to drive mission-focused research. The center aims to cultivate innovative therapies aimed at addressing aging-related diseases, including AMD and cancer. Dr. Seo expressed intentions to foster collaborative research efforts with both domestic and global pharmaceutical firms, which will facilitate the progression of clinical trials for this promising dry AMD therapeutic.
Beyond its immediate implications for AMD treatment, the overarching goal of this research aligns with KIST’s mission to tackle significant national and societal challenges through innovative research and technological advancements. Established in 1966 as Korea’s first government-funded research institute, KIST endeavors to generate sustainable growth engines and address the pressing needs within health care, among other sectors. The implications of this research extend well beyond AMD, potentially influencing the therapeutic landscape for various aging-related conditions characterized by inflammatory processes.
This groundbreaking study received support from the Ministry of Science and ICT, under the leadership of Minister Sang Im Yoo, through KIST’s major projects and the Excellent Young Researcher Program, highlighting the importance of government backing in advancing scientific innovation. The research team’s compelling findings were recognized, resulting in their publication as an inside back cover article in the prestigious international journal "Advanced Science," which boasts an impressive impact factor and ranks among the top journals in its category.
The implications of the newly developed peptide-based eye drops are profound. Not only do they offer a refreshing alternative to patients who may hesitate to undergo traditional injection-based therapies, but they also reflect the ongoing evolution of drug delivery systems within the context of ophthalmic health. As the landscape of AMD treatment continues to develop, the integration of peptide technology into established treatment protocols has the potential to significantly alter the course of disease management and improve quality of life for countless individuals facing vision loss.
The enthusiasm surrounding the research aligns with a global push toward innovative therapies capable of addressing the multifaceted challenges posed by age-related diseases. As AMD remains a crucial public health concern, this novel approach could serve as a model for the development of other targeted therapeutics aimed at similar debilitating conditions, embodying the spirit of advancement that defines modern biomedical research.
As the research team prepares for the next steps, including potential clinical trials, the momentum generated by their groundbreaking work is expected to inspire further investigations into peptide-based therapies across various medical disciplines. This could herald a new era of treatment options, fostering hope for patients who rely on effective interventions to maintain their vision and overall well-being as they age.
In summary, the development of peptide-based eye drops for dry AMD not only marks a significant scientific advancement but also symbolizes the broader efforts to innovate and improve patient-centered care in the field of ophthalmology. The continued collaboration between research institutions and pharmaceutical companies will undoubtedly play a crucial role in bringing this promising therapeutic closer to clinical application, with the potential to transform how AMD and related conditions are treated in the future.
Subject of Research: Development of peptide-based eye drops for dry age-related macular degeneration (AMD)
Article Title: Massively Parallel Screening of Toll/Interleukin-1 Receptor (TIR)-Derived Peptides Reveals Multiple Toll-Like Receptors (TLRs)-Targeting Immunomodulatory Peptides
News Publication Date: 31-Oct-2024
Web References: Advanced Science DOI
References: Korea Institute of Science and Technology, Ministry of Science and ICT
Image Credits: Korea Institute of Science and Technology
Keywords
AMD, peptide therapy, eye drops, Toll-like receptors, inflammation, drug delivery, retinal degeneration, therapeutic innovations, ophthalmology, aging-related diseases, clinical trials, biomedical research.