In a groundbreaking advancement that could dramatically reshape the diagnosis and treatment of certain kidney diseases, a team of researchers has identified recessive genetic variants in the intergenic NOS1AP-C1orf226 locus as the cause of a monogenic form of kidney disease. This discovery, detailed in a comprehensive study published in Nature Communications, opens significant new therapeutic avenues, particularly emphasizing the disease’s surprising responsiveness to anti-proteinuric treatments. This revelation shines a spotlight on the intricate molecular mechanisms underlying kidney function and disease, offering hope to countless patients worldwide contending with unexplained nephropathies.
The study’s core focuses on the intergenic region situated between the NOS1AP (Nitric Oxide Synthase 1 Adaptor Protein) and C1orf226 genes, a region not previously implicated in monogenic kidney disorders. By applying whole-exome sequencing and advanced genetic screening methods across multiple affected families, the researchers pinpointed recessive variants in this locus that lead to kidney dysfunction characterized by proteinuria—a hallmark symptom indicating abnormal kidney filtration. This signifies a paradigm shift, as monogenic causes of proteinuria have been traditionally linked to variants in genes directly coding for structural glomerular proteins, rather than intergenic elements.
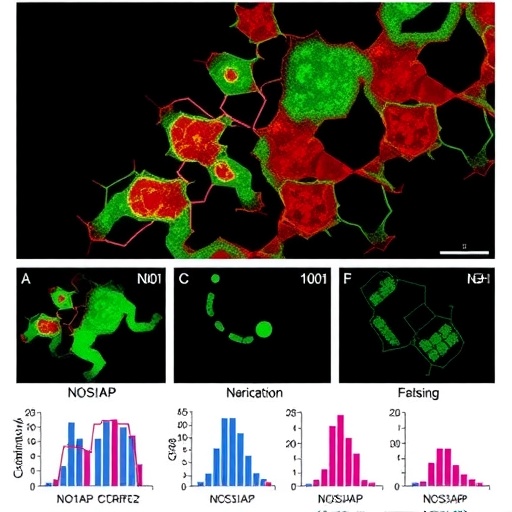
From a molecular biology perspective, the NOS1AP gene encodes a protein known to interact closely with neuronal nitric oxide synthase, modulating nitric oxide signaling pathways, which are crucial in many physiological contexts. C1orf226, on the other hand, remains relatively uncharacterized but is increasingly thought to play a role in cellular functions pertinent to kidney health. The researchers hypothesize that variants in the intergenic NOS1AP-C1orf226 locus disrupt regulatory elements, such as enhancers or promoters, which, in turn, dysregulate gene expression impacting kidney cell homeostasis. This kind of non-coding region pathogenicity highlights the expanding frontier of functional genomics.
Clinically, affected individuals presented with symptoms typical of nephrotic syndrome, especially persistent proteinuria, alongside progressive kidney impairment. However, genetic analyses excluded known causative mutations, necessitating a broader genetic exploration. It was through meticulous linkage studies and whole-genome approaches that the recessive variants in NOS1AP-C1orf226 emerged as consistent and exclusive pathogenic candidates. The recessive inheritance pattern also has profound implications for genetic counseling and predictive testing within affected families.
Therapeutic investigation revealed that this monogenic form of kidney disease notably responds to anti-proteinuric treatment, such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), treatments that are widely used to reduce protein loss in various kidney diseases but often yield inconsistent benefits in monogenic disorders. The responsiveness observed here not only improves clinical prognosis but also suggests that despite genetic causation, modulation of downstream pathophysiological pathways can be therapeutically exploited.
This phenomenon urges a reconsideration of the dogma that monogenic kidney diseases are invariably refractory to conventional pharmacological interventions. The study thereby advocates for the integration of personalized medicine approaches that combine genetic diagnosis with therapy tailored to molecular pathology, potentially extending treatment benefits to other genetic kidney diseases with similar mechanistic disruptions.
The researchers further employed in vitro assays and animal models to validate the pathogenicity of the identified variants. Functional experiments demonstrated that the mutations lead to altered NOS1AP and possibly C1orf226 expression patterns, which affect signaling cascades governing cellular filtration barrier integrity. These preclinical models provide compelling evidence that these variants cause cellular and molecular dysfunction directly correlating with the clinical phenotype, reinforcing the causative link.
On a broader scale, this work underscores the importance of investigating non-coding regions of the genome, traditionally considered “dark matter” in genetics, in the pathology of human diseases. As whole-genome sequencing becomes more accessible, such discoveries will likely proliferate, enriching our understanding of the genetic architecture of diseases beyond classical coding mutations.
Furthermore, the study’s multidisciplinary methodology—blending genomics, clinical nephrology, molecular biology, and pharmacology—exemplifies the future direction of medical research. This integrative approach ensures that findings are not only academically enlightening but also translatable into tangible therapeutic strategies that can alleviate patient suffering.
Importantly, the identification of a treatable monogenic kidney disease challenges current diagnostic algorithms and encourages the early use of comprehensive genetic testing in patients with unexplained proteinuria. Timely diagnosis and initiation of anti-proteinuric therapy could forestall irreversible kidney damage and delay progression to end-stage renal disease, representing a major triumph for patient care.
The impact of this discovery also extends to the realm of drug development. Understanding the molecular pathways implicated in these novel intergenic variants may identify new pharmacological targets. It opens the door to designing precision therapies that could modulate the specific dysfunctional elements caused by the genetic alteration, beyond the traditionally employed broad-spectrum anti-proteinuric agents.
Moreover, this research holds significant relevance for the broader nephrology community, as proteinuria is a common and prognostically important feature across a spectrum of kidney diseases. Improved mechanistic insights afford clinicians deeper diagnostic clarity and reinforce the importance of genetic profiling in patients with atypical presentations.
In addition to the clinical and molecular implications, this study contributes valuable information to the growing compendium of genotype-phenotype correlations in kidney diseases. By cataloging the clinical spectrum associated with NOS1AP-C1orf226 variants, the research delineates a novel disease entity that can now be recognized and differentiated from other hereditary nephropathies.
The societal implications are profound, as kidney diseases impose substantial health care burdens globally. Advances such as these, which translate genetic discoveries into effective treatments, not only improve individual patient outcomes but also have the potential to reduce long-term health care costs associated with chronic kidney disease management and dialysis.
In conclusion, the discovery of recessive variants in the intergenic NOS1AP-C1orf226 locus as the cause of a monogenic kidney disease responsive to anti-proteinuric treatment represents a major leap forward. It highlights the power of genomic medicine to unravel the complexities of kidney pathology and paves the way for targeted therapies that could revolutionize patient management. As research continues to delve into the non-coding genome and its regulatory elements, similar transformative insights are expected to emerge, further bridging the gap between genetic knowledge and clinical practice.
Subject of Research:
Genetic variants in the intergenic NOS1AP-C1orf226 locus causing monogenic kidney disease and their responsiveness to anti-proteinuric treatment.
Article Title:
Recessive variants in the intergenic NOS1AP-C1orf226 locus cause monogenic kidney disease responsive to anti-proteinuric treatment.
Article References:
Buerger, F., Salmanullah, D., Liang, L. et al. Recessive variants in the intergenic NOS1AP-C1orf226 locus cause monogenic kidney disease responsive to anti-proteinuric treatment. Nat Commun 16, 10654 (2025). https://doi.org/10.1038/s41467-025-65663-6
Image Credits: AI Generated
DOI: https://doi.org/10.1038/s41467-025-65663-6