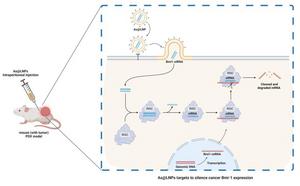
Malignant tumors continue to pose one of the most significant threats to human health and longevity. These neoplasms are not just a personal challenge for those affected but represent a profound global public health crisis. The increasing prevalence of cancer underscores the urgent need for innovative therapeutics that can effectively target and eradicate tumor cells while minimizing damage to healthy tissues. In this context, the recent development of a novel nucleic acid nanomedicine named AS1411-siRNA-LNPs, or As@LNPs, has shown promising potential in treating malignancies through a unique combination of targeted delivery mechanisms and therapeutic agents.
The foundation of the As@LNPs design hinges on the strategic incorporation of Bmi-1 siRNA, a novel therapeutic molecule pivotal for silencing genes associated with cancer cell proliferation. This specific siRNA was encapsulated within cationic liposomes, which serve to protect the RNA from degradation while enhancing cellular uptake. The liposomal formulation is critical not just for stability but also for improving the bioavailability of the drug once administered in the biological environment. These dimensions make cationic liposomes a clever vehicle for targeted drug delivery to cancer cells.
Embedding an aptamer such as AS1411 within the liposome structure further amplifies the nanomedicine’s efficacy. The AS1411 aptamer possesses a unique ability to selectively bind to nucleolin, a protein overexpressed on the surface of many cancer cells. This selective binding is not merely an enhancement but constitutes a targeted approach to therapy by ensuring that the payload of siRNA is delivered efficiently to tumor sites, minimizing exposure to healthy cells. By conjugating AS1411 to the liposome exterior, researchers are capitalizing on the specific interactions between the aptamer and tumor cells, which could lead to higher therapeutic indices compared to conventional treatments.
Upon synthesis, the physicochemical properties of the As@LNPs were meticulously characterized. With an average particle size of 183 nm, As@LNPs are optimized for cellular uptake while remaining small enough to evade the immune system’s recognition. The polydispersity index of 0.187 indicates a uniform distribution of particle sizes, which is crucial for predictable pharmacokinetics and biodistribution. Moreover, an encapsulation efficiency of 85% and a drug loading of 4.6% accentuate the capability of the liposomes to carry a substantial amount of therapeutic cargo, enhancing their effectiveness while reducing the frequency of administration necessary for clinical efficacy.
An intriguing addition to the characterization of As@LNPs is the measurement of average electron mobility, which stood at 2.64 (μ/s)/(V/cm). This metric may correlate with the intracellular behavior of the nanomedicine and could provide insights into its transport mechanisms once internalized by target cells. Zeta potential measurements yielded a value of 33.79 ± 0.78 mV, suggesting a stable dispersion in physiological conditions. Stability is paramount in drug formulation, particularly in ensuring that the therapeutic agents remain intact and effective throughout their lifecycle until they reach their intended target.
A thorough evaluation of the microstructure of As@LNPs was performed using transmission electron microscopy (TEM). TEM images revealed the well-formed morphology of the nanocarrier, providing visual confirmation of the successful construction of the liposomal structure. This analysis is critical in confirming that the intended design of the nanomedicine aligns with the actual product, particularly in nanomedicine, where small-scale deviations can drastically affect functionality and efficacy.
In both in vitro and in vivo experimental models, As@LNPs have illustrated significant anticancer properties. The results showed that these nanomedicines not only inhibited tumor growth but also incited apoptosis among malignant cells. The mechanisms through which As@LNPs exert their effects are multifactorial, involving both the silencing of crucial oncogenes and the modulation of tumor microenvironments that favor cancer cell survival. Such findings underscore the translational potential of As@LNPs from preclinical studies to eventual clinical applications aiming to treat cancers effectively.
Going a step further, the safety profile of As@LNPs was also rigorously assessed. While promising therapeutic outcomes were observed, it is vital to understand the biosafety parameters related to any new treatment modality. As@LNPs presented favorable safety results concerning major tissues and organs, indicating that the therapeutic approach minimizes off-target effects. Nonetheless, caution is warranted as some renal tissues, including glomeruli and renal epithelial cells, indicated potential toxicity, a finding that necessitates further investigation into its biocompatibility.
The dual nature of As@LNPs offers a glimpse into the future of cancer treatment, where targeted therapy meets the precision of gene silencing techniques. This innovative approach could represent a paradigm shift in how malignant tumors are treated by not only addressing the tumors’ robustness through their genetic underpinnings but also doing so in a manner that respects the surrounding healthy tissues. This strategic targeting may pave the way for reduced side effects often associated with conventional chemotherapy.
Moreover, the successful application of nanomedicine such as As@LNPs in clinical settings could catalyze the development of a new class of treatment options tailored specifically to individual patient’s tumor profiles. As research continues to advance, the anticipation of personalized medicine approaches based on genetic and molecular profiling becomes increasingly viable. The goal is a more effective, less toxic treatment landscape for patients battling these complex diseases, ultimately aiming to improve overall survival rates and quality of life.
As the scientific community observes the results of As@LNPs closely, the implications for wider application abound. Researchers are already considering the potential adaptations of this system for other types of RNA interference strategies or even the combination of several therapeutic modalities. The cooperative interaction between nucleic acids, biocompatible delivery systems, and targeted ligands presents an exciting frontier in cancer therapy, which could be leveraged further for chronic diseases beyond malignancies.
The advancement of AS1411-siRNA-LNPs underscores the extraordinary potential of nanotechnology in medicine, particularly oncology. This multidisciplinary approach, weaving together expertise from molecular biology, materials science, and chemistry, exemplifies how innovative thinking can push the boundaries of conventional therapeutics. As we stand on the brink of a new era in cancer therapy, the continuous research and development of such novel formulations could redefine not just how we treat cancer but also how we understand and interact with these life-threatening illnesses.
By investing in comprehensive studies of AS1411-siRNA-LNPs, researchers are paving the way for a robust pipeline of future cancer therapies that harness the innate mechanisms of gene regulation while ensuring enhanced delivery and reduced collateral damage to patients. The hope is that with persistent exploration and validation, solutions will emerge that can transform the narrative of cancer from fear to one of empowerment through choice—offering patients new chances for effective treatments designed specifically for them.
Subject of Research: Novel Nucleic Acid Nanomedicine in Cancer Therapy
Article Title: Targeting Tumors with AS1411-siRNA-LNPs: A New Hope in Cancer Treatment
News Publication Date: Not specified
Web References: Not specified
References: Not specified
Image Credits: Not specified
Keywords: Nucleic acid nanomedicine, cancer therapy, AS1411, siRNA, liposomes, targeted delivery, tumor growth inhibition, biosafety, personalized medicine, gene silencing.