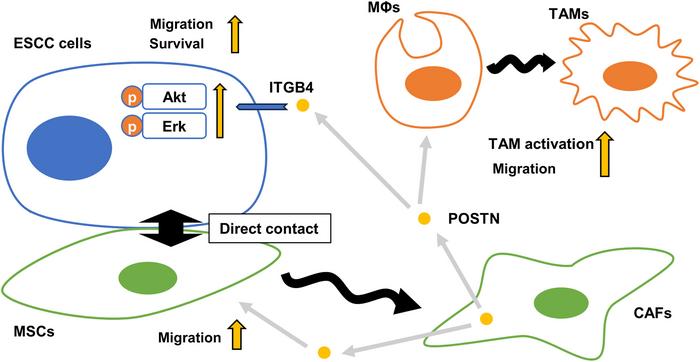
Philadelphia, April 9, 2024 – Esophageal squamous cell carcinoma (ESCC) accounts for around 90% of esophageal cancers, especially in East Asia. New findings in The American Journal of Pathology, published by Elsevier, indicate that periostin, or POSTN, promotes ESCC progression by enhancing cancer and stromal cell migration in cancer-associated fibroblasts (CAFs). Therefore, it may be a novel therapeutic target for treating ESCC.

Credit: The American Journal of Pathology
Philadelphia, April 9, 2024 – Esophageal squamous cell carcinoma (ESCC) accounts for around 90% of esophageal cancers, especially in East Asia. New findings in The American Journal of Pathology, published by Elsevier, indicate that periostin, or POSTN, promotes ESCC progression by enhancing cancer and stromal cell migration in cancer-associated fibroblasts (CAFs). Therefore, it may be a novel therapeutic target for treating ESCC.
Lead investigator Yu-ichiro Koma, MD, PhD, Division of Pathology, Department of Pathology, Kobe University Graduate School of Medicine, explained, “Though CAFs in the tumor microenvironment are involved in the progression of various cancers, including ESCC, the underlying mechanisms are unclear. Therefore, it is critical to further investigate the mechanisms of esophageal cancer development and progression.”
To better understand the mechanisms of ESCC progression by CAFs, investigators generated CAF-like cells by directly coculturing human bone marrow–derived mesenchymal stem cells (MSCs) with ESCC cells, revealing elevated periostin expression. Recombinant human periostin activated the serine/threonine protein kinase (Akt) and extracellular signal-regulated kinase (Erk) signaling pathways in ESCC cells, which enhanced the survival and migration of these cancer cells. Periostin also enhanced MSC and macrophage migration and conferred tumor-associated macrophage (TAM)-like properties to macrophages. Immunohistochemistry demonstrated the clinical significance of periostin, associating its high expression with tumor invasiveness, vessel invasion, advanced pathological stage, CAF marker expression, and TAM infiltration. After direct coculture, ESCC cells showed increased characteristics of malignancy, such as tumor survival, growth, and migration, as well as increased phosphorylation of Akt and Erk.
Dr. Koma commented: “We discovered that periostin, up-regulated in CAFs upon direct contact with cancer cells, promotes ESCC progression and the migration of stromal cells like CAFs and TAMs. Periostin also enhanced mesenchymal stem cell and macrophage migration and endowed macrophages with tumor-associated macrophage-like properties. Thus, CAF-secreted periostin contributed to tumor microenvironment development.”
Dr. Koma concluded: “The present study established a direct coculture system between ESCC cells and MSCs, which promoted the malignant phenotype of ESCC cells. Patients with ESCC with high periostin expression exhibited poorer postoperative outcomes, indicating that periostin may be a novel therapeutic target for treating this form of esophageal cancer and may serve as a prognostic indicator.”
Esophageal cancer is the seventh most common cancer worldwide and the sixth leading cause of cancer-related death. The most common histological subtype is ESCC. The histologic types of esophageal cancer are broadly classified into ESCC and esophageal adenocarcinoma, with ESCC accounting for approximately 90% of esophageal cancers, especially in East Asia. In East Asia and East Africa, esophageal cancer is one of the top five causes of overall cancer mortality, and the five-year overall survival of ESCC is approximately 20%, with a poor prognosis. This prognosis is associated with a high propensity for metastasis, even if the tumor is superficial, Early esophageal cancer is often asymptomatic and diagnosis typically occurs at an advanced stage.
Journal
American Journal Of Pathology
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Periostin in Cancer-Associated Fibroblasts Promotes Esophageal Squamous Cell Carcinoma Progression by Enhancing Cancer and Stromal Cell Migration