Johns Hopkins Medicine researchers have made a pivotal discovery regarding the epigenetic modifications that occur in pancreatic cells during their transition from a normal state to one that is intertwined with cancerous characteristics. Their study, which highlights a significant relationship between inflammation and cell identity transformation, underscores how normal pancreatic cells can harbor a form of “memory” of cancer-associated epigenetic marks, suggesting a precancerous potential even before genetic mutations occur. The findings, reported in the prestigious journal Genome Medicine, open new avenues for understanding pancreatic cancer, a disease notorious for its challenging prognosis and late-stage diagnosis.
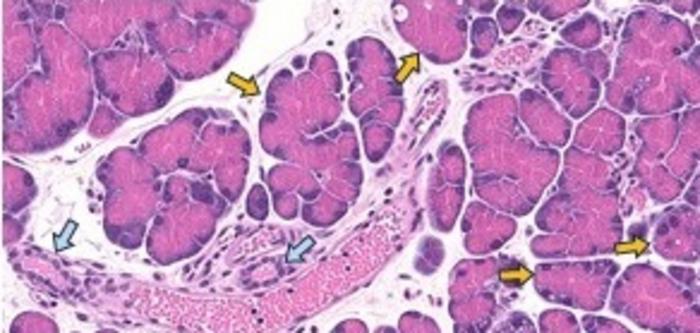
The transition of acinar cells, which are responsible for producing digestive enzymes, into ductal cells—a process known as acinar-to-ductal metaplasia—emerges from an inflammatory pancreatic environment. This transformation serves as a protective mechanism for acinar cells, enabling them to withstand inflammatory damage. This biological adaptability highlights a critical adaptive strategy within cellular biology, offering insights into how normal tissues can evolve under pathological conditions while retaining certain epigenetic signatures linked to malignancy.
Epigenetic modifications refer to the chemical alterations that influence gene expression without changing the underlying DNA sequence. The analogy of DNA as a computer’s hardware juxtaposed with the epigenome as its software aptly illustrates this dynamic. Gene expression is precisely orchestrated by the presence or absence of these epigenetic marks, which can dictate cell identity and function. The research focused specifically on how these modifications impact pancreatic cells as they navigate between normal and diseased states, thus providing an in-depth understanding of the epigenetic landscape in a cancer-prone environment.
In this study, led by Andrew Feinberg, M.D., a distinguished figure in epigenetics at Johns Hopkins, the researchers utilized advanced genomic sequencing techniques to explore the complete epigenetic landscape of pancreatic cells undergoing this crucial transitional phase. Their investigations yielded remarkable results—epigenetic markers were identified on genes associated with pancreatic cancer, specifically within the PI3K and R/R/C GTPase pathways. Notably, these findings emerged in the absence of the genetic mutations commonly observed in human pancreatic precursor lesions, thereby establishing a unique case of epigenetic transformation devoid of mutagenic events.
The implications of these findings are profound. They suggest that pancreatic cells can adopt a predisposed state towards malignancy due to reversible epigenetic changes that bear resemblance to those seen in precancerous conditions. The recognition of this pathway of transformation highlights the influence of non-genetic factors, such as inflammation and cellular stress, in initiating cancer-related transitions, which could shift how we approach cancer prevention and intervention strategies, especially in younger patients who lack age-related genetic mutations.
Moreover, the retention of some epigenetic markers even after the cells reverted back to their acinar form indicates a form of cellular memory. This aspect is particularly intriguing because it suggests that surviving inflammatory events could imprint long-lasting changes in gene regulation, predisposing cells to neoplastic transformations in the future. This epigenetic memory could potentially serve as a biomarker for early diagnosis of pancreatic cancer, providing a window for intervention before clinical manifestations occur.
The study also propels forward the argument that the increasing incidence of pancreatic cancer among younger demographics might not solely be attributed to genetic predispositions. Instead, the transition states shaped by inflammatory environments could significantly contribute to the observed shift in cancer prevalence, thereby altering the landscape of risk assessment and preventive measures in oncology.
As researchers continue to explore the depth of epigenetic alterations and their ramifications on cellular identity, it is imperative to integrate these findings into developing novel therapeutic strategies. Understanding the mechanism by which normal cellular processes can exploit epigenetic pathways to veer into a pathological state is key for devising interventions that could reverse or prevent such transitions.
Another essential component of this research is the concerted contribution of a multidisciplinary team at Johns Hopkins, including Emily Lo, who served as the first author, and Patrick Cahan, Ph.D., all of whom collaborated seamlessly to illuminate this intricate intersection of epigenetics and cancer biology. Their collective effort fosters a deeper understanding of the cellular networks involved in the evolution of pancreatic cancer, emphasizing the need for targeted research initiatives that encompass varied biological disciplines.
Ultimately, this pioneering exploration of pancreatic cell transitions not only accentuates the complexity of cancer biology but also fosters hope for innovative diagnostic and therapeutic strategies that could transform the approach to pancreatic cancer management, an area that remains obscenely challenging. As knowledge progresses, the potential for decoding the epigenetic language of cells could herald a new era in combating this insidious disease that has long eluded effective treatment.
The research highlights that while genetic mutations undoubtedly play a role in the emergence of cancer, the epigenetic changes occurring during transitional states warrant particular attention. By elucidating these modifications, scientists may be better equipped to devise strategies that can either block the conversion of normal cells to cancerous ones or revert already altered cells back to a healthy state.
This research and its implications could significantly alter the public health landscape regarding pancreatic cancer. As the scientific community continues to unravel the layers of epigenetic regulation and its consequences, there is potential not only for advancing our understanding of cancer mechanisms but also for initiating practices that could lead to earlier detection and more personalized treatment approaches for patients.
Subject of Research: Epigenetic alterations in pancreatic cancer development
Article Title: Johns Hopkins Research Illuminates Epigenetic Pathways in Pancreatic Cancer
News Publication Date: March 28, 2023
Web References: Genome Medicine
References: DOI
Image Credits: Credit: Johns Hopkins Medicine
Keywords: Pancreatic cancer, epigenetics, acinar-to-ductal metaplasia, inflammatory response, cellular transformation, cancer biology, gene regulation, pancreatic precursors.