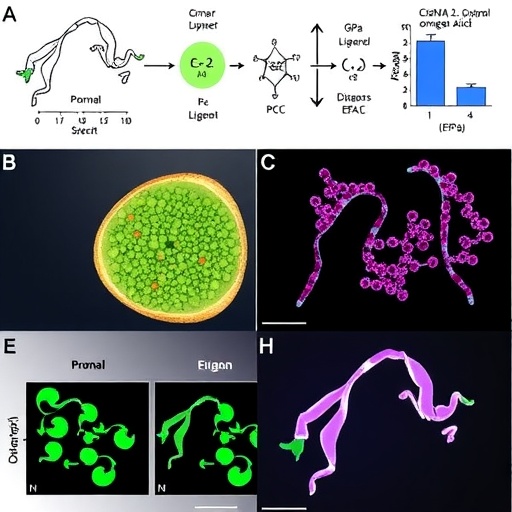
In the intricate landscape of mammalian biology, the mammary gland stands out as a marvel of developmental adaptability and functional precision. Throughout lactation, this organ undergoes remarkable changes, enabling it to produce and secrete milk—a vital source of nutrition and immune protection for neonates. While it is established that dietary components such as omega-3 polyunsaturated fatty acids (PUFAs), including docosahexaenoic acid (DHA), positively influence lactation, the precise molecular pathways mediating these effects have remained elusive. Recent groundbreaking research has now illuminated a sophisticated signaling cascade involving G protein-coupled receptors (GPCRs) that directly governs mammary development and lipogenesis beyond the established anti-inflammatory mechanisms.
GPCRs comprise a vast and diverse family of transmembrane receptors pivotal for cellular responses to external stimuli. Among these, G protein-coupled receptor 120 (GPR120) and GPR40 are recognized sensors of long-chain fatty acids. They are known to exert anti-inflammatory effects predominantly via the GPR120–β-arrestin2 signaling axis. However, the question remained whether their roles in mammary physiology are solely tied to inflammation modulation or if they propagate additional, direct developmental and metabolic effects essential for lactation.
The research team addressed this gap by investigating the impact of omega-3 fatty acids on lactation performance under non-inflammatory conditions. Their observations demonstrated that supplementation with n-3 PUFAs enhanced lactational output irrespective of inflammation status, strongly implying the existence of a novel, direct molecular regulatory mechanism. Further examination revealed that GPR120 is not only expressed in the mammary gland but exhibits a unique lactation-specific upregulation pattern, with expression levels progressively increasing across the lactation timeline. This temporal correlation pointed to an intrinsic, functionally significant role for GPR120 in mammary epithelial physiology.
Mechanistic investigations revealed that n-3 fatty acids activate GPR120 located on the membranes of mammary epithelial cells, initiating a signaling cascade through the stimulatory G protein alpha subunit (Gαs). This activation leads to a surge in cyclic adenosine monophosphate (cAMP) levels, triggering downstream effectors in a pathway independent of the typical anti-inflammatory routes. Central among these effectors is the exchange protein directly activated by cAMP (EPAC), which serves as a critical node bifurcating into parallel regulatory circuits influencing both lipid metabolism and tissue development.
At the metabolic level, EPAC activates the chromatin architectural protein CTCF, a master regulator of the spatial organization of chromatin. By remodeling chromatin landscapes, CTCF facilitates the assembly of transcription factor hubs centered on peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT/enhancer-binding protein alpha (C/EBPα). These transcription factors orchestrate the upregulation of genes encoding key enzymes and transporters essential for milk lipid biosynthesis, notably fatty acid synthase (FASN) and fatty acid translocase (CD36). Consequently, this pathway enhances lipogenesis—the synthesis of milk fats—and promotes the accumulation of lipid droplets within mammary epithelial cells, bolstering milk quality and quantity.
Simultaneously, the EPAC pathway modulates mammary gland morphogenesis. EPAC induces the secretion of the chemokine CXCL14 by mammary epithelial cells in an autocrine manner. This chemokine, through its receptor CXCR4, establishes a self-sustaining CXCL14-CXCR4 loop critical for guiding the polarized migration of epithelial cells. This migration is instrumental in the formation of acinar structures—the milk-producing units of the mammary gland—via activation of two key intracellular signaling pathways: AKT (protein kinase B) and ERK (extracellular signal-regulated kinase). The concerted action of these cascades drives epithelial organization and differentiation, essential for maintaining functional gland architecture during lactation.
The unveiling of this “GPR120-EPAC dual axis” represents a paradigm shift in our understanding of mammary biology. It highlights a sophisticated molecular mechanism by which n-3 PUFAs directly regulate both the developmental and metabolic dimensions of the mammary gland. This mechanism operates independently of previously known anti-inflammatory pathways, underscoring the nuanced complexity of receptor-mediated signal transduction in tissue function.
Moreover, the identification of the autocrine CXCL14 signaling loop as a key driver of acinar formation advances the frontier of developmental biology within the context of lactation. It also opens up avenues for exploring chemokine-mediated regulation of epithelial morphogenesis, a concept that may extend beyond the mammary gland to other organs with glandular structures.
Clinically, these findings carry substantial implications. Enhancing maternal lactational ability remains a global health priority, given the critical role of breastfeeding in infant development. Targeting components of the GPR120–Gαs–cAMP–EPAC signaling axis or modulating the CXCL14-CXCR4 loop could lead to innovative interventions designed to improve milk production and mammary health. Dietary supplementation strategies could be optimized to leverage this molecular pathway, offering tangible benefits for maternal and neonatal outcomes.
In addition to its physiological significance, this work spotlights the broader utility of GPCRs in regulating complex biological functions. The detailed elucidation of the signaling cascade from extracellular fatty acid sensing to epigenetic and transcriptional reprogramming exemplifies the integrative nature of cellular communication networks. It underscores the importance of considering signaling pathways in their full cellular and molecular context when devising therapeutic strategies.
The study also contributes to a deeper comprehension of how environmental and nutritional cues translate into tissue-specific developmental programs. As diet-derived molecules engage receptor systems and intracellular effectors to modulate gene expression and cell behavior, this research underscores the molecular dialogue between metabolism and development. Such insights are quintessential as biomedical science endeavors to decipher and manipulate the mechanisms governing organ function and adaptation.
Future research building on these findings may explore the potential cross-talk between GPR120 and other fatty acid receptors, the dynamic regulation of EPAC isoforms in mammary epithelial cells, and the interplay between metabolic and immune signaling during lactation. Deciphering how these pathways integrate with hormonal regulation and systemic physiological cues will provide an even more comprehensive understanding of mammary gland biology.
In sum, this research elegantly bridges nutritional biochemistry with cellular signaling and developmental biology, revealing a nuanced mechanism whereby omega-3 fatty acids exert a dual regulatory role on mammary gland lipogenesis and morphogenesis. It propels the understanding of lactation physiology into a new era, marked by the identification of precise molecular targets and the potential for novel clinical applications to enhance maternal and neonatal health worldwide.
Subject of Research: Not applicable
Article Title: Omega-3 Fatty Acids Regulate Mammary Gland Lipogenesis and Development via Gαs-Mediated cAMP–EPAC Signaling Pathway
News Publication Date: 8-Jul-2025
Web References: http://dx.doi.org/10.34133/research.0767
Image Credits: Copyright © 2025 Baofeng Li et al.
Keywords: Omega-3 fatty acids, GPR120, mammary gland, lactation, lipogenesis, EPAC signaling, CXCL14, cAMP, GPCR, mammary development, acinar formation, lipid metabolism