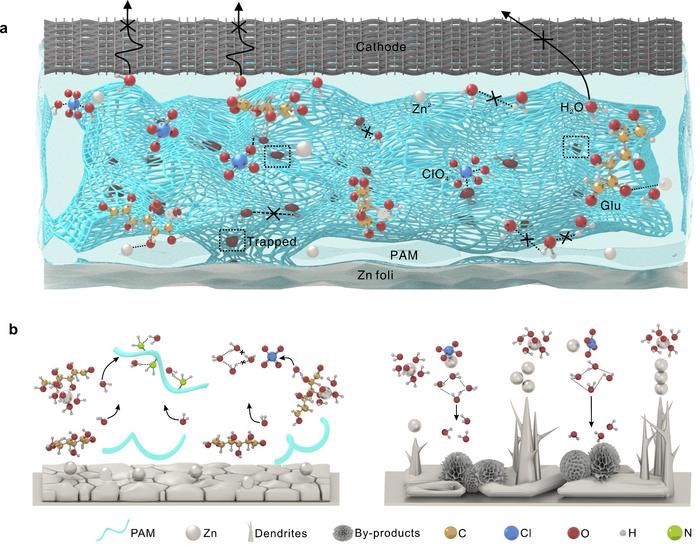
According to a study published in Advanced Energy Materials, a research team led by Prof. HU Linhua from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences has constructed a hydrogel electrolyte formula by using ClO4– anions and polyacrylamide chains to anchor water molecules, while glucose molecules preferentially regulate Zn2+ solvation.

Credit: LI Zhaoqian
According to a study published in Advanced Energy Materials, a research team led by Prof. HU Linhua from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences has constructed a hydrogel electrolyte formula by using ClO4– anions and polyacrylamide chains to anchor water molecules, while glucose molecules preferentially regulate Zn2+ solvation.
Effectively interrupted water clusters and enhanced water covalency were realized, resulting in an expanded voltage stability window and stable operation over a wide temperature range.
“This means that the aqueous zinc batteries could operate stably considering the seasonal and altitude factors. Importantly, the temperature resistance mechanism in the water environment, Zn2+ solvation and Zn/electrolyte interface are systematically analyzed,” said LI ZHAO Qian, a member of the team.
Irreversible electrolyte phase transitions and an accelerated parasitic reaction greatly threaten the climate adaptability of aqueous Zn-ion batteries. Water activity affects the freezing point of the electrolyte, the voltage stability window, and interfacial Zn deposition behavior. Due to its anti-leakage property, polymer structure stability, and numerous anchoring sites for free water, the hydrogel electrolyte’s rational design efficiently improves the battery’s climate adaptability.
In this study, the researchers construct a “covalency reinforced” hydrogel electrolyte with superior interfacial adhesion and strong moisture-retaining ability. Through spectral analysis and theoretical calculations, they revealed weakened bulk water activity and regulated Zn2+ solvation, which delayed the freezing point of the electrolyte, facilitated its moisture-retaining capacity, and inhibit water-induced side reactions.
COMSOL simulation and morphological evolution show the improved mechanical properties of the electrolyte and the thermodynamically stable Zn interface. These advantages resist dendrite formation and solve electrode–electrolyte contact problems, giving the batteries a wide operating range of -40~130°C.
“When the electrolyte is used in pouch batteries, it shows an impressive capacity of 254 mAh/g at -30°C and 438.1 mAh/g at room temperature. This is a big deal because most previous batteries didn’t go beyond 200 mAh/g at -30°C or 400 mAh/g at room temperature. This work shows how effective these batteries are, both in terms of capacity and their ability to operate over a wide range of temperatures,” said Dr. LI.
They also assembled the Zn//Zn and Zn//Cu batteries to evaluate stable lifespan and Zn plating/stripping reversibility. At low current density, the lifetime of the Zn anode exceeds 2,000 hours, which is better than that of the liquid electrolyte. Even at high current density, the battery with Glu/ZC/PAM can work steadily for more than 500 hours. The Zn//Cu batteries could work steadily for more than 800 hours with a high average Coulomb efficiency of 99.2%, highly competitive with previous hydrogel electrolytes.
This study modulates the coordination structure and tailors thermodynamic activity between the electrolyte/Zn interface by employing a multifunctional hydrogel electrolyte, which degenerates detrimental parasitic reactions and extends the operating temperature range. It provides a safe and highly efficient strategy to realize all-climate aqueous zinc-ion devices.
Journal
Advanced Energy Materials
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Regulating Water Activity for All-Climate Aqueous Zinc-Ion Batteries
Article Publication Date
12-Jun-2024