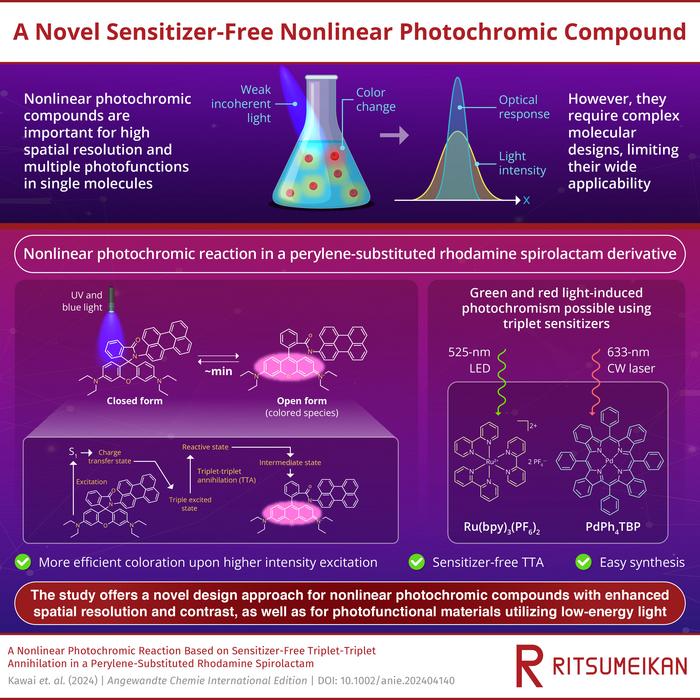
Photochromic compounds, which change their color when exposed to light, have been widely used as photoswitches to control different properties of materials. Nonlinear photochromic compounds, characterized by a nonlinear response to the intensity of incident light, have attracted special attention among researchers as the nonlinearity leads to enhanced contrast and improved spatial resolution in photochromic reactions. It also allows for multiple photochromic properties in a single molecule with a single light source. These qualities have made them valuable in nonlinear optical and holographic elements, super-resolution microscopy, and biomedical applications.

Credit: You are free to share and adapt the material. Attribution is required.
Photochromic compounds, which change their color when exposed to light, have been widely used as photoswitches to control different properties of materials. Nonlinear photochromic compounds, characterized by a nonlinear response to the intensity of incident light, have attracted special attention among researchers as the nonlinearity leads to enhanced contrast and improved spatial resolution in photochromic reactions. It also allows for multiple photochromic properties in a single molecule with a single light source. These qualities have made them valuable in nonlinear optical and holographic elements, super-resolution microscopy, and biomedical applications.
The simplest way to achieve nonlinear photochromism in materials is through simultaneous two-photon absorption, but this requires extremely high-intensity light. For nonlinear photochromic reactions using low-intensity light, stepwise two-photon processes are required, but these are difficult to design because they rely on extremely short-lived molecular species. Moreover, multi-photochromic systems that exhibit stepwise photochemical reactions require complex molecular structures. Such complexities have limited the wide applications of nonlinear photochromic compounds in many fields. Another important method for inducing nonlinear photochromism is triplet-triplet annihilation (TTA). It requires three components: a triplet sensitizer, an annihilator, and a photochromic compound, which adds significant complexity. If a single molecule can fulfill these roles, nonlinear photochromism can be achieved in simpler systems.
In a recent breakthrough, a team of researchers from Japan, led by Professor Yoichi Kobayashi from the Department of Applied Chemistry at the College of Life Sciences at Ritsumeikan University, achieved nonlinear photochromism with low-intensity light using TTA in a single molecule. Prof. Kobayashi explains, “Rhodamine spirolactams can play the roles of both photochromic compounds and triplet sensitizers, which addresses the issue of complexity, and they can be easily synthesized from rhodamine B and its analogs. In this study, we focused on a rhodamine spirolactam derivative having a perylene group (Rh-Pe) and investigated its photochromic properties.” Their findings were published in the journal Angewandte Chemie International Edition on April 10, 2024.
In Rh-Pe, excitation with light triggers a photochromic reaction, leading to the formation of a ring-opening structure, called open form, which results in drastic changes in its color. Upon studying the nonlinear photochromic properties of Rh-Pe, the researchers found that the color change efficiency of Rh-Pe increased with higher-intensity light. This means that the intensity of coloration and thus the generated amount of the open form increases non-linearly with an increase in excitation intensity. For example, on excitation with 365 nm light from a light-emitting diode, Rh-Pe showed almost no color change. However, excitation with a higher-intensity 355-nm nanosecond pulse laser resulted in significant coloration, even though the light had lower energy in total.
To understand the origin of these nonlinear photochromic properties, the researchers studied the excitation mechanism of Rh-Pe. They found that when directly excited with ultraviolet (UV) and blue light, Rh-Pe transitions into a charge-transfer state, which then produces a triplet excited state. This triplet excited state then undergoes TTA, forming an intensely colored open form via an intermediate state. This TTA accounts for the nonlinear response to light intensity since it works more efficiently with higher-intensity light. Additionally, the researchers demonstrated that Rh-Pe can also exhibit photochromism with red and green light-induced photochromism by using separate triplet sensitizers, even though it can be directly excited by UV and blue light.
“Our novel design for easily synthesized nonlinear photochromic compounds will pave the way for their diverse applications, such as high-resolution photolithography, 3D printing, and high-density optical disks,” says Prof. Kobayashi, emphasizing the significance of their findings. “Our results offer a novel approach for the design of photochromic compounds and functional materials with nonlinear behavior and long-wavelength responsivity that efficiently utilize low-energy light.”
Overall, the findings of the study offer new avenues for developing simpler nonlinear photochromic compounds, paving the way for their wider applications.
***
Reference
About Ritsumeikan University, Japan
Ritsumeikan University is one of the most prestigious private universities in Japan. Its main campus is in Kyoto, where inspiring settings await researchers. With an unwavering objective to generate social symbiotic values and emergent talents, it aims to emerge as a next-generation research university. It will enhance researcher potential by providing support best suited to the needs of young and leading researchers, according to their career stage. Ritsumeikan University also endeavors to build a global research network as a “knowledge node” and disseminate achievements internationally, thereby contributing to the resolution of social/humanistic issues through interdisciplinary research and social implementation.
Ritsumeikan University Research Report:
About Professor Yoichi Kobayashi from Ritsumeikan University, Japan
Dr. Yoichi Kobayashi is a Professor in the Department of Applied Chemistry in Ritsumeikan University, Japan, and a Ritsumeikan Advanced Research Academy (RARA) Associate Fellow. Dr. Kobayashi graduated from Kwansei Gakuin University, Japan, in 2007, from where he also obtained his PhD in 2011. Before joining Ritsumeikan University, he worked for the Japan Society for the Promotion of Science at the University of Toronto, Canada, and Aoyama Gakuin University for several years. He now leads a research group in the Photofunctional Physical Chemistry Lab, where they conduct cutting-edge studies on photochromism, optical nanostructures and nanoparticles, photophysics, and photochemistry. He has published over 70 peer-reviewed papers.
Funding information
This work was supported by JST, PRESTO Grant Numbers JPMJPR22N6, JSPS KAKENHI Grant Number JP18H05263, JP21K05012, JP23H01940, JP23H04878 in a Grant-in-Aid for Transformative Research Areas “Materials Science of Meso-Hierarchy”, and the Sasakawa Scientific Research Grant from The Japan Science Society.
Journal
Angewandte Chemie International Edition
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
A Nonlinear Photochromic Reaction Based on Sensitizer-Free Triplet-Triplet Annihilation in a Perylene-Substituted Rhodamine Spirolactam
Article Publication Date
10-Apr-2024
COI Statement
The authors declare no conflict of interest.