A new frontier in cancer therapy is emerging from the collaborative efforts of scientists at the University of Massachusetts Amherst and Ernest Pharmaceuticals. Their research focuses on a novel non-toxic bacterial treatment, referred to as BacID, designed to enhance the delivery of cancer-fighting drugs directly into tumors. Unlike conventional chemotherapy, which often subjects patients to harsh side effects, this innovative approach aims to provide a safer and more effective alternative by harnessing the unique properties of genetically engineered bacteria.
The promising technology targets a range of aggressive cancers, including liver, ovarian, and metastatic breast cancers, which are historically associated with poor prognoses. The research team has dedicated over a decade to refining BacID, and they are now poised to take an important step forward, with clinical trials set to commence in 2027. Neil Forbes, the senior author of the study published in the esteemed journal Molecular Therapy, expressed optimism about the developments, stating that they now have the necessary components to advance bacterial-based cancer treatments that are likely more effective and user-friendly.
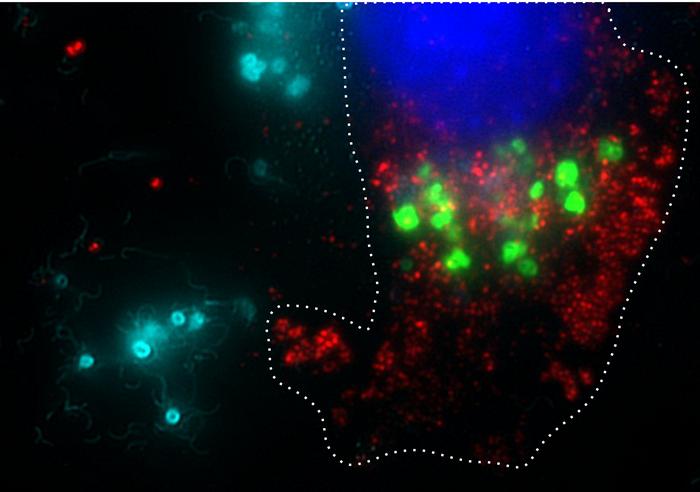
The core mechanism of BacID involves genetically modified strains of Salmonella that are engineered to selectively target tumor cells while sparing healthy tissues from damage. Bacteria possess a natural proclivity for homing in on tumors, which researchers are keen to exploit. This characteristic not only allows for localized treatment but also means that the engineered bacteria can proliferate significantly within tumor environments, delivering a greater concentration of therapeutic agents than traditional systemic delivery methods could provide.
One of the key breakthroughs of this research lies in the modulation of bacterial activity via the administration of aspirin. The engineering team discovered that the bacterial flagella, which enable motility, are critical for the invasion of cancer cells. By designing a genetic circuit that activates flagella production upon aspirin intake, the bacteria remain dormant until the patient ingests the common medication. This controlled activation presents significant advantages, as it allows for precise timing in the cancer cell invasion process.
Aspirin’s role as a turn-on switch significantly enhances the safety profile of the treatment. Without the aspirin activation, the modified bacteria remain quiescent within the body, only springing into action once they are in the vicinity of the tumor and the medication is taken. This adds a level of patient agency to the treatment, allowing individuals to self-administer an over-the-counter drug that triggers the therapy three days after the initial bacterial infusion.
Moreover, the research addresses previous limitations in the delivery systems used in earlier iterations of bacterial therapy. Prior models required the bacteria to navigate blindly to tumors, which resulted in risks of healthy tissue invasion and premature elimination of bacteria before they could effectively colonize tumors. This new approach optimizes the bacteria’s capability to invade tumor cells safely and efficiently, minimizing potential adverse effects while maximizing therapeutic delivery.
The engineered bacteria also incorporate a built-in suicide mechanism, designed to rupture as they release the therapeutic agents within cancer cells. This ingenious design not only enhances the efficacy of the treatment but ensures that the bacteria do not linger in the body beyond their usefulness. The precise control over their lifecycle allows for optimal therapeutic windows and reduces the likelihood of off-target effects, which have been a significant concern with traditional therapies.
Early studies utilizing mouse models have illustrated the effectiveness of the BacID treatment paradigm. In these preclinical trials, after intravenous injection, the modified bacteria demonstrated a tendency to proliferate predominantly within tumors while being rapidly eliminated from healthy tissues by the immune system. This selective growth pattern is decisively advantageous, as it significantly augments the dosage available directly at the tumor site without burdening the patient’s overall health.
Looking forward, the research team is actively preparing for regulatory approval processes that will set the stage for human clinical trials. Their goal is not only to validate the safety and efficacy of BacID in human populations but also to establish comprehensive protocols that will bring this technology from the laboratory bench to the clinic. This translational research is critical for ensuring that breakthrough discoveries reach patients who can benefit from them and underscores the importance of rigorous scientific investigation in medical advancements.
BacID also showcases the monumental potential in harnessing living organisms for therapeutic use as part of the growing field of microbiome-based treatments. The interplay between cancer biology and microbial engineering might hint at a revolutionary future where similar approaches can be developed for various medical applications beyond oncology, which could further bolster the repertoire of safe and effective treatment options available to patients.
In combination with ongoing advancements in precision medicine, this approach offers hope for more personalized cancer treatment strategies. The intricate design of BacID aligns with the principles of molecular targeting, aiming to enhance therapeutic efficacy while minimizing systemic toxicity. As this exciting research progresses, stakeholders across the medical and scientific communities remain eager to observe the innovative developments that may redefine oncological care.
With the culmination of years of diligent research and engineering, the advances made by the UMass Amherst-Ernest Pharmaceuticals team may mark a pivotal moment in the evolution of cancer treatments. By prioritizing patient safety and treatment efficacy, this innovative approach to drug delivery using genetically modified bacteria may set a paradigm shift in how we manage some of the most challenging cancers known today.
As the trial date approaches, there remains a palpable sense of optimism within the research community, fueled by the collective belief that targeted bacterial therapies like BacID could illuminate new pathways for cancer treatment and ultimately lead to better outcomes for patients battling these dangerous diseases.
Subject of Research: Cancer Therapy Using Genetically Engineered Bacteria
Article Title: Controlling intracellular protein delivery, tumor colonization and tissue distribution using the master regulator flhDC in a clinically relevant ΔsseJ Salmonella strain
News Publication Date: 30-Dec-2024
Web References: Molecular Therapy DOI link
References: Molecular Therapy Journal
Image Credits: UMass Amherst
Keywords: BacID, cancer therapy, Salmonella, drug delivery, aspirin-triggered treatment, genetic engineering, clinical trials, targeted therapy, tumor targeting, non-toxic treatment, microbial treatment, precision medicine.