Osaka, Japan – Antisense oligonucleotides (ASOs) are next-generation drugs that can treat disease by blocking the transfer of harmful messages from our genes. In people with cancer, ASOs have the potential to block messages that encourage the growth and spread of the tumor. However, ASOs aren’t used for treating cancer yet. They must first get delivered inside cancer cells, but the cancer cells won’t let them in.
Finding an effective ASO delivery system is a major challenge. Cancer cells have gatekeeper molecules that stop unwanted substances from entering. Although investigators have tried many ways of getting ASOs past the gatekeepers, success has been limited.
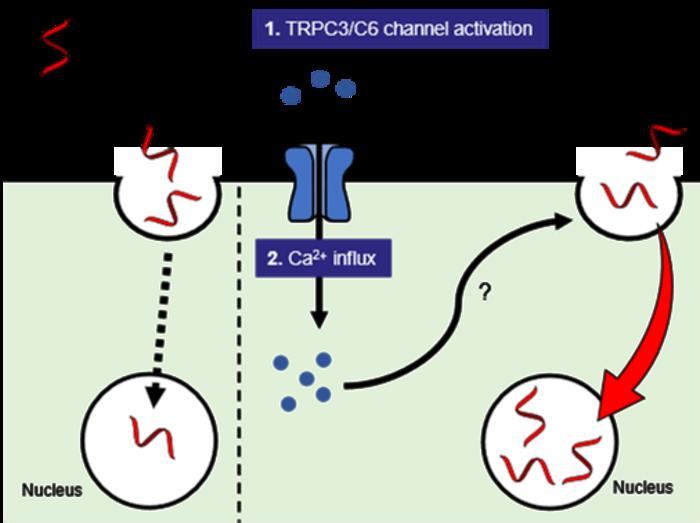
Now, in a study recently published in the journal Nucleic Acids Research, researchers from Osaka University have discovered a way to deliver ASOs to their targets inside cancer cells. The team synthesized a new compound, named L687, which opens specific calcium permeable channels on the surface of cancer cells. When the calcium flows into cells through the open channels it tells the cells to let in the ASOs.
“We discovered that we could selectively activate the TRPC3/C6 calcium permeable channels1) with the activator L687,” explains lead author Hiroto Kohashi. “We then found that combination treatment with L687 and ASO promoted efficient uptake of ASO into cancer cells during laboratory tests and tumor cells inside the mouse. As a result, target gene activity was suppressed and ASO efficacy was enhanced.”
Until now, ASOs have mainly been used to treat incurable diseases and had to be delivered into the liver or spinal fluid. According to the Osaka team’s research, L687 is an effective drug delivery system that may extend the benefits of ASO treatment to other parts of the body.
“We hope that the results of our research will lead to significant progress in the development and delivery of ASOs and similar gene-targeting drugs for treating cancer,” says senior author Masahito Shimojo.
The team believes that L687 could be a particularly effective way of delivering ASO therapy to lung or prostate cancers. These cancers have many TRPC3/C6 calcium permeable channels1) that can be opened by L687, potentially revealing new targets for these next-generation therapies.
1) TRPC3/C6 channels belong to a Transient Receptor Potential Canonical (TRPC) Channel subfamily of a TRP channel superfamily.
###
The article, “A novel transient receptor potential C3/C6 selective activator induces the cellular uptake of antisense oligonucleotides,” was published in Nucleic Acids Research at DOI: https://doi.org/10.1093/nar/gkae245.

Credit: 2024 Nagata et al., A novel transient receptor potential C3/C6 selective activator induces the cellular uptake of antisense oligonucleotides., Nucleic Acids Research
Osaka, Japan – Antisense oligonucleotides (ASOs) are next-generation drugs that can treat disease by blocking the transfer of harmful messages from our genes. In people with cancer, ASOs have the potential to block messages that encourage the growth and spread of the tumor. However, ASOs aren’t used for treating cancer yet. They must first get delivered inside cancer cells, but the cancer cells won’t let them in.
Finding an effective ASO delivery system is a major challenge. Cancer cells have gatekeeper molecules that stop unwanted substances from entering. Although investigators have tried many ways of getting ASOs past the gatekeepers, success has been limited.
Now, in a study recently published in the journal Nucleic Acids Research, researchers from Osaka University have discovered a way to deliver ASOs to their targets inside cancer cells. The team synthesized a new compound, named L687, which opens specific calcium permeable channels on the surface of cancer cells. When the calcium flows into cells through the open channels it tells the cells to let in the ASOs.
“We discovered that we could selectively activate the TRPC3/C6 calcium permeable channels1) with the activator L687,” explains lead author Hiroto Kohashi. “We then found that combination treatment with L687 and ASO promoted efficient uptake of ASO into cancer cells during laboratory tests and tumor cells inside the mouse. As a result, target gene activity was suppressed and ASO efficacy was enhanced.”
Until now, ASOs have mainly been used to treat incurable diseases and had to be delivered into the liver or spinal fluid. According to the Osaka team’s research, L687 is an effective drug delivery system that may extend the benefits of ASO treatment to other parts of the body.
“We hope that the results of our research will lead to significant progress in the development and delivery of ASOs and similar gene-targeting drugs for treating cancer,” says senior author Masahito Shimojo.
The team believes that L687 could be a particularly effective way of delivering ASO therapy to lung or prostate cancers. These cancers have many TRPC3/C6 calcium permeable channels1) that can be opened by L687, potentially revealing new targets for these next-generation therapies.
1) TRPC3/C6 channels belong to a Transient Receptor Potential Canonical (TRPC) Channel subfamily of a TRP channel superfamily.
###
The article, “A novel transient receptor potential C3/C6 selective activator induces the cellular uptake of antisense oligonucleotides,” was published in Nucleic Acids Research at DOI: https://doi.org/10.1093/nar/gkae245.
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan’s leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world, being named Japan’s most innovative university in 2015 (Reuters 2015 Top 100) and one of the most innovative institutions in the world in 2017 (Innovative Universities and the Nature Index Innovation 2017). Now, Osaka University is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website:
Journal
Nucleic Acids Research
Method of Research
Experimental study
Subject of Research
Animals
Article Title
A novel transient receptor potential C3/C6 selective activator induces the cellular uptake of antisense oligonucleotides
Article Publication Date
16-Apr-2024