Understanding the intricate mechanics behind how our brains function remains one of the most profound challenges in neuroscience. The processes governing thought, emotion, memory, and movement all hinge on synaptic transmission—the rapid exchange of chemical signals between neurons. Central to this phenomenon are tiny molecular containers known as synaptic vesicles, which ferry neurotransmitters to precisely timed release sites. Recent advances spearheaded by an international team of scientists have now illuminated the complex vesicle cycle with an unprecedented level of detail, leveraging innovative computational models that simulate the dynamics of these vesicles like never before.
A collaboration between the Okinawa Institute of Science and Technology (OIST) in Japan and the University Medical Center Göttingen (UMG) in Germany has culminated in a groundbreaking study published in Science Advances. By creating a highly detailed spatial model that integrates molecular, cellular, and synaptic level information, these researchers have reconstructed the complete synaptic vesicle cycle. Their model transcends previous limitations, allowing exploration of synaptic behaviors under a variety of conditions, including those difficult or impossible to simulate in laboratory experiments. This represents a transformative step in decoding the cellular orchestration underlying synaptic communication.
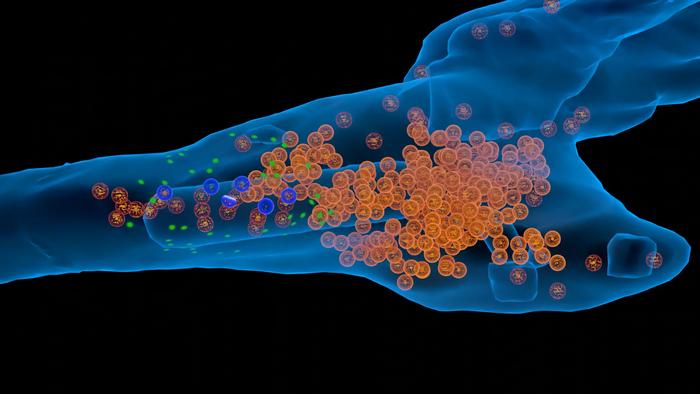
At its core, synaptic transmission is driven by the release of neurotransmitter molecules stored within vesicles—microscopic sac-like structures. These vesicles migrate toward the presynaptic membrane, dock at specific sites known as active zones, and fuse with the membrane in response to electrical stimulation. This fusion event liberates neurotransmitters into the synaptic cleft, where they engage receptors on the postsynaptic neuron, propagating the neural signal. Following release, vesicles undergo complex recycling pathways, preserving synaptic function and sustainability. Although the broad outline of this cycle has been known, many mechanistic details—especially regarding spatial organization and molecular interactions—have remained elusive until now.
The computational simulation employed by the team incorporates an intricate spatial representation of synaptic components. This includes the clustering of vesicles into distinct pools: the recycling pool, which supplies vesicles ready for immediate use, and the reserve pool, an immobilized cluster serving as a repository for replenishment. Notably, only about 10 to 20 percent of vesicles reside in the recycling pool at any time, emphasizing the critical regulatory mechanisms governing vesicle mobilization and availability. The model quantitatively describes how vesicles transit between these pools in response to synaptic activity, providing insight into the molecular underpinnings orchestrating this balance.
One of the most striking revelations from the model is the synaptic vesicle cycle’s remarkable capacity to sustain function at stimulation frequencies far exceeding those typically observed in vivo. This finding challenges prior conceptions of synaptic limitations and opens new perspectives on synaptic resilience under extreme physiological or pathological states. The ability of vesicle cycling to maintain rapid, continuous neurotransmitter release even during high-frequency firing underscores the robustness of synaptic machinery and its finely tuned regulatory processes.
Critical to this robustness is the role of specific proteins such as synapsin-1 and tomosyn-1, whose regulatory effects emerge clearly from the model. Synapsin-1 is implicated in tethering vesicles to the reserve pool, acting as a molecular anchor that controls vesicle availability. Tomosyn-1 influences the release probability by modulating vesicle priming and fusion readiness. The model’s ability to simulate the dynamics of these proteins and their interactions with vesicle pools sheds light on fundamental molecular mechanisms that govern synaptic efficiency and plasticity.
Molecular tethering emerges as a pivotal mechanism within the vesicle cycle, as elucidated by the modeling. Tethers physically link vesicles to the cell membrane, ensuring that a rapid supply of vesicles is accessible to docking sites. This physical proximity reduces waiting times for vesicle docking and fusion, thereby facilitating sustained neurotransmitter release during periods of intense neural activity. The spatial modeling of these tethering interactions represents a novel aspect of synaptic simulation, offering unprecedented resolution into vesicle dynamics at nanometer scales.
The implications of these findings extend beyond pure neuroscience, touching upon medical fields concerned with neurological disorders. Disruptions in vesicle cycling and neurotransmitter release are implicated in a range of pathologies—from botulism and myasthenic syndromes, where toxin interference impedes vesicle exocytosis, to depression and psychiatric disorders, many of which involve altered synaptic transmission. By providing a detailed computational platform, this study offers a tool for probing how molecular dysfunctions translate into synaptic failure, paving the way for targeted therapeutic interventions.
Professor Erik De Schutter, head of the Computational Neuroscience Unit at OIST and co-author of the study, highlights the transformative potential of integrated modeling approaches. He notes that the exponential growth in experimental data necessitates sophisticated tools to unify and make sense of disparate datasets. Their simulation bridges molecular mechanisms and cellular outcomes with computational efficiency and spatial precision, marking progress toward the ambitious goal of full-cell and eventual full-tissue computational simulation in neuroscience.
From a methodological standpoint, the researchers combined cutting-edge imaging data, biophysical measurements, and molecular biology with high-performance computational resources. This integrated approach enabled the incorporation of diverse datasets into a cohesive, dynamic model. The flexibility of the model allows it to be adapted to different types of cells and experimental conditions, enhancing its utility across various research domains.
Professor Silvio Rizzoli, director at UMG and co-author, reflects on the significance of having a predictive computational framework. For decades, experimental limitations constrained direct testing of synaptic function at fine temporal and spatial scales. This model permits hypothesis testing about vesicle dynamics and synaptic behavior under conditions that extend beyond experimental reach, especially in the context of neurological diseases. It represents a collaboration of experimental and theoretical neuroscience yielding tangible advancements.
Future directions include expanding the model to simulate synaptic interactions within larger networks, exploring how vesicle dynamics influence neural circuit function and behavior. Integration with molecular pathways involved in disease states may further shed light on pathogeneses and aid the development of novel pharmaceuticals. The versatility and depth of the model promise broad impact, not only enhancing fundamental understanding but also accelerating translational neuroscience.
In summary, this pioneering computational exploration of the synaptic vesicle cycle provides an unprecedented window into the molecular choreography that supports rapid and sustained communication between neurons. By simulating the full spatial and molecular complexity of vesicle pools, tethering mechanisms, and protein regulation, the study pushes the boundaries of what can be achieved with integrative neuroscience approaches. Its insights hold profound implications for biology, medicine, and the future of brain research.
Subject of Research: Cells
Article Title: Dynamic Regulation of Vesicle Pools in a Detailed Spatial Model of the Complete Synaptic Vesicle Cycle
News Publication Date: 28-May-2025
Web References: 10.1126/sciadv.adq6477
Image Credits: Gallimore et al., 2025
Keywords: Synaptic vesicle cycle, neurotransmitter release, computational modeling, vesicle tethering, synapsin-1, tomosyn-1, synaptic transmission, neuronal communication, dynamic regulation, vesicle pools, hippocampal synapses, high-frequency stimulation