In a groundbreaking interdisciplinary study, researchers at the University of Maryland, Baltimore County (UMBC) have unveiled new complexities underlying the movement of cells through biological tissues, shedding light on the intricate interplay between chemical cues and the physical structure of tissues. Utilizing the fruit fly egg chamber as a model system, the team’s work, recently published in iScience, harnesses advanced mathematical modeling alongside state-of-the-art imaging techniques to decode how cells navigate their environment — a discovery with far-reaching implications for understanding developmental biology, cancer metastasis, and tissue regeneration.
Cell migration is a fundamental biological process, critical to embryonic development, immune system function, and wound repair. Traditionally, the prevailing view emphasized chemical gradients as the primary drivers of cellular movement, where cells migrate in response to steadily increasing concentrations of chemoattractant molecules. However, the UMBC team’s research challenges this notion by demonstrating that the physical architecture of the tissue environment dramatically modulates cellular migration patterns. The fruit fly egg chamber, a well-established experimental system, serves as a convincing model because of its analogous cellular dynamics to mammalian systems and accessibility for both biological and mathematical exploration.
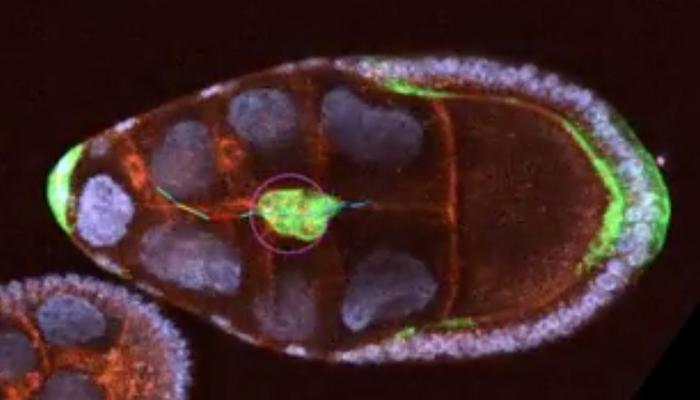
The study focuses on border cells within the fruit fly egg chamber, specialized migratory cells whose movement is governed by chemical signals from their surrounding milieu. Traditionally conceived as cells migrating up a chemical gradient, border cells were found to respond instead to a more nuanced combination of chemoattractant distribution shaped by tissue geometry. The egg chamber’s complex landscape, characterized by alternating narrow tubules and wider gaps, influences how chemical signals disperse, creating heterogeneous cues that alter migratory speed and directionality. This underscores the critical role of biophysical constraints in shaping cellular behavior.
Biologist Alex George, a key contributor to the study, explains that the migration path taken by border cells resembles the fairy tale of Hansel and Gretel following breadcrumbs through a dense forest. On flat, uniform terrain, chemical cues would gradually intensify, providing straightforward guidance. However, in the irregular topography of the egg chamber, chemoattractants accumulate unevenly, resembling pools of breadcrumbs accumulating unpredictably in valleys and ravines. This nuanced environment challenges cells to interpret complex signals rather than simply following a steady chemical gradient.
To delve deeper into this phenomenon, the research team developed sophisticated mathematical models that simulate cell movement by integrating the effects of both chemical signal distribution and tissue architecture. Naghmeh Akhavan, a mathematical biologist on the team, crafted these models to quantitatively capture how physical constraints impact the dispersion of chemoattractants and, consequently, border cell velocity. The models predict that cells accelerate in narrow tubules, where chemical cues become concentrated, and decelerate in wider gaps where signals disperse and weaken. These theoretical predictions were confirmed experimentally by George’s advanced imaging techniques.
This fusion of experimental data and computational modeling stands out as a paradigm of interdisciplinary research. Unlike previous studies that prioritized either chemical signaling or physical morphology, this investigation represents one of the first efforts to explicitly quantify how these two factors co-regulate cell migration. The iterative feedback loop between wet-lab experimentation and modeling refined both approaches, resulting in a robust framework capable of capturing the complex, dynamic realities of cell behavior in vivo. “Our model revealed subtle patterns invisible to traditional methods,” said Akhavan, “and seeing our theoretical outcomes mirrored in real biological systems was truly exhilarating.”
Furthermore, the research employed cutting-edge microscopy at the Advanced Imaging Center at the Janelia Research Campus in Virginia, where specialized instruments captured previously elusive dynamics of chemoattractant molecules in living tissue. These high-resolution temporal and spatial data provided the empirical foundation for refining the mathematical constructs, enabling the team to simulate realistic biological conditions. This level of precision imaging marks a significant advancement in visualizing the molecular microenvironment of migrating cells, paving the way for deeper insights into cellular navigation mechanisms.
The implications of these findings extend well beyond developmental biology. Cell migration underpins critical physiological and pathological processes, including immune surveillance, tissue repair, and the spread of cancer cells during metastasis. Understanding how cells integrate competing cues from their environment to modulate movement has the potential to transform therapeutic strategies aimed at controlling undesirable cell migration. For example, manipulating tissue geometry or chemical gradients could become a novel approach to limiting cancer invasiveness or enhancing wound healing efficacy.
UMBC biologist Michelle Starz-Gaiano, also a co-author, emphasizes that this research addresses a fundamental gap in cell migration studies by illustrating the interdependence of chemical and structural cues. “Most prior investigations treated these influences in isolation,” she notes. “Our data-driven insights open new avenues for designing medical interventions that consider the holistic microenvironment in which cells operate, potentially unlocking more effective treatments.”
As the research team continues to build upon this foundation, their focus increasingly targets innovative experimental designs and more refined mathematical models. The integration of these methodologies promises to unveil additional layers of complexity inherent in cell migration, including how variations in tissue stiffness or extracellular matrix composition might further diversify migratory behaviors. The dynamic between biological inquiry and quantitative analysis highlights a transformative approach for future studies in cell physiology.
Looking ahead, the team’s collaborative efforts exemplify how interdisciplinary synergy is essential for addressing biological phenomena that defy reductionist explanations. By bridging mathematics, biology, and advanced imaging, their study underscores the emerging necessity to transcend traditional disciplinary boundaries to unravel the sophisticated language cells use to interpret their environment. This research not only marks a milestone in our understanding of chemotaxis and tissue geometry interaction but also sets a new standard for how complex biological questions should be approached.
In summary, the UMBC team has articulated a novel conceptual framework in which tissue geometry shapes the spatial distribution of chemoattractants, which in turn governs the speed and migratory patterns of border cells in the fruit fly egg chamber. This pivotal advancement reveals that cells do not simply respond to chemical signals in a linear fashion but rather interpret spatially complex, geometry-influenced landscapes of signals. Such insights refine our fundamental conception of cellular navigation and hold profound promise for biomedical applications aiming to control cellular motility in diverse contexts.
Subject of Research: Cells
Article Title: Chemotaxis of Drosophila border cells is modulated by tissue geometry through dispersion of chemoattractants
News Publication Date: 21-Mar-2025
Web References:
https://www.sciencedirect.com/science/article/pii/S2589004225002196
References:
DOI: 10.1016/j.isci.2025.111959
Image Credits: Michelle Starz-Gaiano
Keywords:
Cell migration, Cellular physiology, Cell behavior, Metastasis, Mathematical modeling