In a groundbreaking advancement that promises to deepen our understanding of metabolic disorders, researchers have successfully elucidated the cryo-electron microscopy (cryo-EM) structures of human Isovaleryl-Coenzyme A Dehydrogenase (IVD), unveiling the sophisticated mechanisms underlying its substrate specificity and the molecular repercussions of disease-associated mutations. IVD serves a critical role in the catabolism of the branched-chain amino acid leucine, catalyzing the conversion of isovaleryl-CoA into 3-methylcrotonyl-CoA. Deficiencies in this enzyme’s function result in the accumulation of toxic metabolites, causing isovaleric acidemia (IVA), a severe autosomal recessive disorder characterized by metabolic acidosis, vomiting, and neurological impairment. For years, precise structural insights into IVD have been limited, hampering the development of targeted therapies. Now, these newly resolved high-resolution structures illuminate the enzyme’s architecture, substrate engagement, and the mechanistic basis of pathogenic mutations.
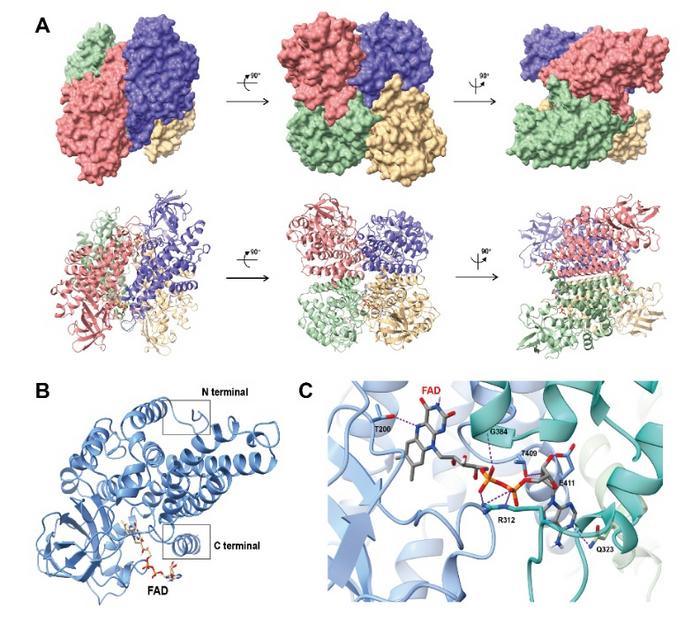
At the core of this research is the remarkable revelation that human IVD assembles as a tetramer, with each monomer adopting a distinctive “U-shaped” substrate channel formed through the interplay of α-helices and β-sheet domains. This unique geometry governs substrate selectivity, favoring short-chain branched substrates characteristic of leucine metabolism while excluding longer substrates through steric restrictions. These findings were made possible after researchers refined protein purification protocols to capture IVD both in its apoenzyme form and bound to substrates such as isovaleryl-CoA and butyryl-CoA, providing a comparative framework for understanding substrate recognition and catalysis.
Detailed structural analysis further highlights the exceptional specificity of IVD within the broader acyl-CoA dehydrogenase (ACAD) enzyme family. Unlike medium-chain acyl-CoA dehydrogenase (ACADM), which metabolizes straight-chain fatty acyl substrates ranging from six to twelve carbons, IVD selectively interacts with branched substrates typically bearing four to six carbons. At the molecular level, critical residues lining the active site distinguish these enzymes: leucine residues L127 and L290 in IVD constrict the substrate pocket, creating a spatial bottleneck absent in ACADM, where corresponding residues T121 and V284 allow a more spacious active site. This refined architecture ensures that substrates longer than seven carbons cannot be accommodated by IVD, a crucial aspect preventing non-specific activity and ensuring metabolic fidelity.
Comparative structural studies between IVD and ACADM have highlighted additional nuances governing substrate flexibility and specificity. ACADM features bulky residues, such as Y400 and E401, that impose lateral restrictions within the active site, limiting substrate mobility. In contrast, IVD replaces these with glycine and alanine (G406 and A407), residues with minimal side-chain bulk. This substitution further facilitates the accommodation of branched-chain substrates by reducing steric hindrance, showcasing a delicate balance between structural constraints and flexibility. These atomic-level distinctions represent an evolutionary fine-tuning of enzymatic function tailored to metabolic requirements.
The investigation didn’t stop at substrate recognition; it also elucidated the catalytic core of IVD’s activity. Central to its enzymatic mechanism is the glutamate residue E286, which orchestrates the critical abstraction of the α-hydrogen from the substrate, facilitating subsequent biochemical transformations. Moreover, the flavin adenine dinucleotide (FAD) cofactor plays a pivotal role not only in redox chemistry but also in stabilizing the tetrameric assembly through an intricate network of hydrogen bonds involving residues T200, R312, and E411. These interactions are vital for maintaining enzyme integrity and catalytic efficiency.
Crucially, this work sheds new light on the molecular pathology of IVA by revealing how disease-associated mutations perturb enzyme function. Mutations such as A314V and E411K, which have previously been observed clinically, disrupt the delicate equilibrium between structure and function by compromising FAD binding or distorting the substrate-binding pocket. For instance, the E411K mutation substitutes a negatively charged glutamate with a positively charged lysine, undermining cofactor interaction and destabilizing tetramer formation. These disruptions culminate in a greater than 80% reduction in enzymatic activity, offering a direct mechanistic explanation for the severe phenotypes observed in IVA patients.
The implications extend beyond basic science; by correlating specific genotypes with phenotypic outcomes through atomic-level scrutiny, this framework enhances diagnostic precision, allowing clinicians to better anticipate disease progression based on mutation profiles. This structural blueprint paves the way for personalized treatment strategies by identifying mutation-specific vulnerabilities that may be amenable to targeted therapeutic intervention.
Looking ahead, the high-resolution cryo-EM data provide a robust platform for the rational design of small-molecule therapeutics aimed at stabilizing mutant IVD enzymes. By focusing on the FAD-binding region or the substrate pocket, novel compounds might restore partial functionality to defective enzymes, mitigating the toxic metabolic buildups characteristic of IVA. Such pharmacological chaperones could revolutionize treatment paradigms, transitioning from symptomatic management to molecularly targeted therapies.
Moreover, these structural revelations deepen our comprehension of substrate channeling within the enzymatic tetramer and open new investigative avenues into how IVD’s dynamic conformational shifts influence enzyme kinetics and substrate turnover. Understanding these transient states could unveil additional regulatory mechanisms pertinent to metabolic control and disease.
From a broader perspective, this study exemplifies the transformative power of cryo-EM technology in resolving complex enzyme structures that have long eluded researchers due to their dynamic nature and size. It also underscores the importance of integrating structural biology with clinical genetics to unravel the pathophysiology of inherited metabolic disorders, thereby accelerating translational research.
In summary, this comprehensive structural analysis of human IVD not only deciphers the enzyme’s substrate specificity and catalytic machinery but also elucidates how pathogenic mutations disrupt function with devastating clinical consequences. This breakthrough heralds a new chapter in rare metabolic disease research, offering hope for enhanced diagnostic capabilities and innovative therapeutic interventions that directly target the molecular root causes of isovaleric acidemia.
Subject of Research: Not applicable
Article Title: Structural Insights into Isovaleryl-Coenzyme A Dehydrogenase: Mechanisms of Substrate Specificity and Implications of Isovaleric Acidemia-Associated Mutations
News Publication Date: 28-May-2025
Web References: http://dx.doi.org/10.34133/research.0661
Image Credits: Copyright © 2025 Kaide Ju et al.
Keywords: Isovaleryl-CoA Dehydrogenase, Isovaleric Acidemia, Cryo-electron Microscopy, Enzyme Structure, Substrate Specificity, Metabolic Disorders, FAD Cofactor, ACAD Family, Mutation Pathology, Protein Tetramer, Structural Biology, Enzymatic Mechanism