Every great superhero needs a sidekick. Now, scientists may have found a drug-busting partner for naloxone.

Credit: Emily Moskal/Stanford Medicine
Every great superhero needs a sidekick. Now, scientists may have found a drug-busting partner for naloxone.
Naloxone is an opioid antidote that has saved tens of thousands of lives by rapidly reversing opioid overdoses in more than 90% of cases in which it is used. But its powers are temporary, lasting only 30 to 90 minutes. The rise of potent, long-acting opioids such as fentanyl means that someone brought back from the brink can still overdose after the naloxone wears off.
In a new study, Stanford Medicine scientists and collaborators have discovered a novel compound that can work alongside naloxone, supercharging its life-saving effects.
When tested in mice, adding the compound to a miniscule dose of naloxone made it as powerful as the conventional dosage, with the added benefit of milder withdrawal symptoms.
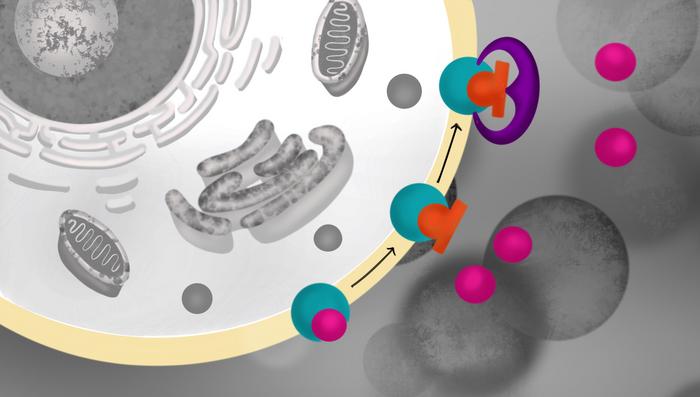
Naloxone, which is given as a nasal spray or injection, works by seizing opioid receptors, kicking out opioids and taking their place. (Naloxone has no addictive properties of its own.) The researchers found that the new compound — known for now as compound 368 — binds next to naloxone on opioid receptors and helpfully holds naloxone in place.
The findings will be published July 3 in Nature.
“Naloxone binding to an opioid receptor turns it mostly off, but not all the way,” said Evan O’Brien, PhD, a postdoctoral scholar in molecular and cellular physiology and the lead author of the new study. “Our data shows that compound 368 is able to increase the binding of naloxone and turn the receptor off more completely.”
The senior authors of the study are Brian Kobilka, MD, professor of molecular and cellular physiology and the Hélène Irwin Fagan Chair in Cardiology at Stanford Medicine; Jay McLaughlin, PhD, professor of pharmacodynamics at the University of Florida College of Pharmacy; and Susruta Majumdar, PhD, professor of anesthesiology at the Washington University School of Medicine in St. Louis.
A new type of drug
The new compound belongs to an unusual class of drugs that don’t directly target the active site on receptors. Instead, they bind elsewhere on the receptor but trigger a structural change that alters the active site. Known as allosteric modulators (allos meaning “other” in Greek), they create new possibilities in drug development, but are trickier to identify, O’Brien said.
“Allosteric modulators are not common yet, and they’re a lot more difficult to discover and to work with,” he said.
Compound 368 is the first known allosteric modulator that can help turn off opioid receptors.
The researchers picked out compound 368 from a library of 4.5 billion compounds. Using advanced high-throughput techniques, they were able to screen the entire molecular library in just two days. To identify potential allosteric modulators that could cooperate with naloxone, they selected for compounds that bind only to receptors already saturated with naloxone.
Compound 368 — an otherwise rather unremarkable compound, O’Brien said — stood out for its ability to tightly bind to opioid receptors only in the presence of naloxone. Like a loyal sidekick, it doesn’t work with other drugs, and it doesn’t work alone.
Powers combined
When researchers exposed cells with opioid receptors to compound 368, they found that the compound alone made little difference. But when cells were given the compound with naloxone, the combination was a powerful deterrent against opioid binding.
The more compound 368 they added, the better naloxone was able to block opioids, including morphine and fentanyl.
“The compound itself doesn’t bind well without naloxone,” O’Brien said. “We think naloxone has to bind first, and then compound 368 is able to come in and cap it in place.”
Indeed, using cryoEM imaging to visualize frozen molecular structures, the researchers found that compound 368 docks right next to naloxone on the opioid receptor, forming bonds that secure the drug in place and slow its natural degradation by the body.
Boosting naloxone
Next, collaborators in McLaughlin’s lab tested the new compound in mice that had been given morphine. Because opioids reduce pain sensation, the researchers observed how quickly a mouse removed its tail from hot water. The stronger the opioid antidote, the faster a mouse would take its tail out of the water.
When mice on morphine were treated with compound 368 alone, nothing changed.
“The compound in mice, at least from the assays we’ve run, does nothing on its own,” O’Brien said. “We don’t observe any off-target effects. We don’t see anything happen to the mice even when we inject a massive amount of compound 368.”
This was exactly what the researchers had predicted from their molecular work and a good sign of the compound’s safety, he added.
When they also gave the mice a small dose of naloxone — an amount that typically would have no effect — the pairing with compound 368 dramatically improved naloxone’s effects.
“When we start to give them more and more of compound 368 with that low dose of naloxone, they take their tail out of the water pretty quickly,” O’Brien said.
Other effects of opioids, such as respiratory depression (the usual cause of death in opioid overdoses), were also reversed by a small dose of naloxone enhanced with the new compound.
Remarkably, the combination of compound 368 with a half dose of naloxone was strong enough to counter fentanyl, which is about 100 times more potent than morphine and the main culprit of overdoses in the United States.
By requiring less naloxone, the new compound could also ease the withdrawal symptoms that opioid users experience after overdose treatment. These symptoms — including body aches, shivering, nausea and diarrhea — are immediate and can be extremely uncomfortable, O’Brien said.
The researchers found that a low dose of naloxone plus compound 368 could reverse the effects of opioids with much milder withdrawal symptoms — in mice, this meant less teeth chattering, jumping and diarrhea.
Saving lives
The team, with the Majumdar lab’s expertise in medicinal chemistry, is now tweaking compound 368 so it can help naloxone counter strong opioids for longer durations.
“We’re still working on optimizing the compound’s properties for those longer-lasting effects,” O’Brien said. “But first showing that it works cooperatively with these low doses of naloxone suggests that we’re on the right track.”
O’Brien is optimistic that this track will lead to trials in humans. Overdoses from synthetic opioids, primarily fentanyl, continue to surge, killing nearly 74,000 Americans in 2022. “The more tools at our disposal, the better we’ll be able to fight this epidemic of fentanyl overdoses,” he said.
Researchers from Kurume University, SLAC National Acceleration Laboratory, Princeton University and University of Copenhagen also contributed to the work.
The study received funding from an American Diabetes Association Postdoctoral Fellowship, an American Heart Association Postdoctoral Fellowship, the National Institutes of Health (grant RO1DA057790) and the Chan Zuckerberg Biohub.
# # #
About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu.
Journal
Nature
Method of Research
Computational simulation/modeling
Subject of Research
Animals
Article Publication Date
3-Jul-2024