“In the present study we describe the development of an inhibitory nanobody directed against an extracellular epitope present in the native V-ATPase c subunit.”

Credit: 2024 Li et al.
“In the present study we describe the development of an inhibitory nanobody directed against an extracellular epitope present in the native V-ATPase c subunit.”
BUFFALO, NY- August 15, 2024 – A new research paper was published in Oncotarget’s Volume 15 on August 14, 2024, entitled, “A nanobody against the V-ATPase c subunit inhibits metastasis of 4T1-12B breast tumor cells to lung in mice.”
The vacuolar H+-ATPase (V-ATPase) is an ATP-dependent proton pump that functions to control the pH of intracellular compartments as well as to transport protons across the plasma membrane of various cell types, including cancer cells.
Researchers Zhen Li, Mohammed A. Alshagawi, Rebecca A. Oot, Mariam K. Alamoudi, Kevin Su, Wenhui Li, Michael P. Collins, Stephan Wilkens, and Michael Forgac from Tufts University School of Medicine; Tufts University; Dana Farber Cancer Institute, Harvard Medical School; University of Minnesota School of Medicine; Prince Sattam Bin Abdulaziz University; Korro Bio; SUNY Upstate Medical University; and Foghorn Therapeutics, have previously shown that selective inhibition of plasma membrane V-ATPases in breast tumor cells inhibits the invasion of these cells in vitro. They have now developed a nanobody directed against an extracellular epitope of the mouse V-ATPase c subunit.
“We show that treatment of 4T1-12B mouse breast cancer cells with this nanobody inhibits V-ATPase-dependent acidification of the media and invasion of these cells in vitro.”
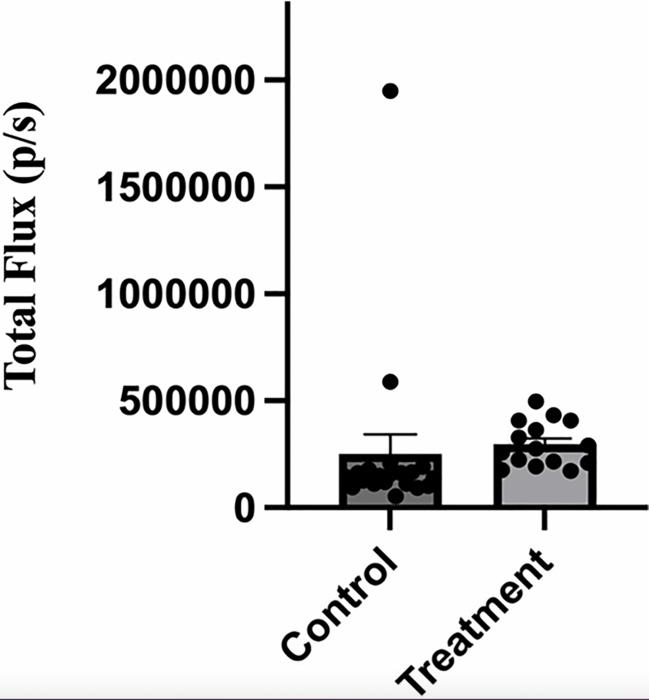
The research team further found that injecting this nanobody into mice implanted with 4T1-12B cells orthotopically in the mammary fat pad inhibited the metastasis of tumor cells to the lungs.
“In conclusion, our results indicate that a nanobody directed against an extracellular epitope expressed on the surface of the V-ATPase is able to inhibit activity of cell surface V-ATPases in 4T1-12B breast cancer cells, inhibit in vitro invasion of these cells and inhibit metastasis of these cells to lung following their implantation in the mammary fat pad of mice.”
Correspondence to: Michael Forgac – michael.forgac@tufts.edu
Keywords: vacuolar ATPase; breast cancer; invasion; tumor metastasis; tumor growth
Click here to sign up for free Altmetric alerts about this article.
About Oncotarget:
Oncotarget (a primarily oncology-focused, peer-reviewed, open access journal) aims to maximize research impact through insightful peer-review; eliminate borders between specialties by linking different fields of oncology, cancer research and biomedical sciences; and foster application of basic and clinical science.
Oncotarget is indexed and archived by PubMed/Medline, PubMed Central, Scopus, EMBASE, META (Chan Zuckerberg Initiative) (2018-2022), and Dimensions (Digital Science).
To learn more about Oncotarget, visit Oncotarget.com and connect with us on social media:
X
Facebook
YouTube
Instagram
LinkedIn
Pinterest
Spotify, and available wherever you listen to podcasts
Click here to subscribe to Oncotarget publication updates.
For media inquiries, please contact media@impactjournals.com.
Oncotarget Journal Office
6666 East Quaker St., Suite 1
Orchard Park, NY 14127
Phone: 1-800-922-0957 (option 2)
Journal
Oncotarget
Method of Research
News article
Subject of Research
Animals
Article Title
A nanobody against the V-ATPase c subunit inhibits metastasis of 4T1-12B breast tumor cells to lung in mice
Article Publication Date
14-Aug-2024
COI Statement
Authors have no conflicts of interest to declare.