In a groundbreaking advancement poised to revolutionize three-dimensional tissue imaging, researchers at Juntendo University in Tokyo have unveiled a novel immunolabeling technique that dramatically accelerates and enhances the visualization of neural and glial structures deep within thick brain tissue. This innovative method, known as POD-nAb/FT-GO 3D-IHC, leverages the unique properties of nanobodies fused with peroxidase enzymes combined with an innovative fluorescent signal amplification system, enabling researchers to achieve ultra-high-resolution 3D imaging within mere days—reducing what traditionally took weeks to a fraction of the time.
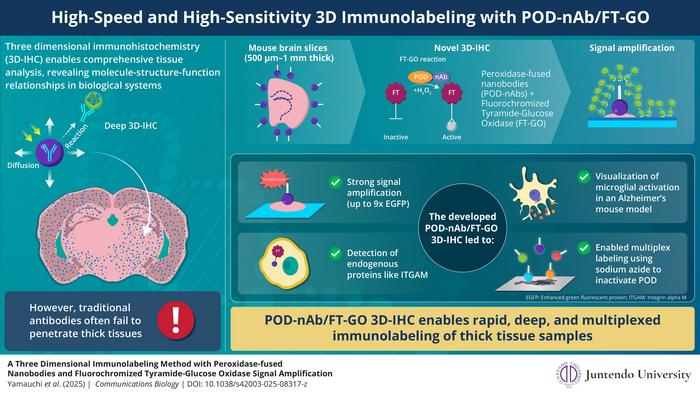
Three-dimensional immunohistochemistry (3D-IHC) has long been celebrated for its capacity to preserve the spatial context of cells and molecules in intact tissue samples. It has become indispensable in neuroscience, pathology, and broader biomedical research for mapping cellular arrangements and interactions. Yet, conventional immunolabeling techniques have faced persistent difficulties in antibody penetration, particularly in tissue samples thicker than a few hundred microns. Their large molecular size and slow diffusion hinder deep labeling, often resulting in superficial staining and limiting the quantitative and qualitative insights achievable.
The team at Juntendo University, led by Assistant Professor Kenta Yamauchi and Professor Hiroyuki Hioki, has countered these challenges by engineering a method that profoundly improves penetration depth and signal sensitivity. Central to the approach are nanobodies—single-domain antibodies derived from camelids—which are approximately one-tenth the size of conventional immunoglobulin antibodies. This small size endows them with drastically improved diffusibility in dense tissue matrices. The researchers further enhanced functionality by fusing nanobodies with horseradish peroxidase (POD), an enzyme capable of catalyzing localized deposition of fluorescently tagged tyramides.
This enzyme-nanobody conjugation was integrated with a fluorescent tyramide signal amplification system named Fluorochromized Tyramide-Glucose Oxidase (FT-GO). The FT-GO system not only amplifies fluorescence signals significantly but also ensures that the labeling process produces high-density, uniform staining throughout the entire depth of thick tissue slices. Optimizing tissue preparation using a urea-based permeabilization reagent called ScaleA2 allowed this system to achieve near-homogeneous labeling across 1-mm-thick mouse brain slices—a milestone far beyond the capabilities of standard antibody methods, which generally fail to penetrate past the superficial layers.
Yamauchi emphasized the technical prowess of the method: “By fusing camelid nanobodies with peroxidase enzymes and pairing them with our original FT-GO signal amplification, we have overcome the longstanding depth limitations of immunohistochemistry. This has allowed for highly sensitive and uniform labeling deep within neural tissues, all achieved in a rapid timeframe.” Such performance makes this approach particularly suited for capturing intricate cellular architectures and protein distributions with unprecedented clarity and speed.
Beyond the technical merits, the technique demonstrated impressive versatility. The researchers successfully labeled both exogenous fluorescent proteins such as EGFP and tdTomato and endogenous markers including integrin alpha M (CD11b), a microglial marker. Uniform labeling throughout deep tissue volumes is a significant leap, especially for studies investigating microglia, which play essential roles in neuroinflammation and neurodegeneration.
An intriguing aspect of this method is the possibility of multiplexed labeling. The team utilized sodium azide (NaN₃) to quench residual POD activity after each staining round, enabling sequential and selective labeling of multiple antigens within the same tissue sample. This multiplexing capacity is crucial for comprehensive tissue mapping, allowing researchers to investigate complex cellular interactions and molecular pathways simultaneously.
The POD-nAb/FT-GO system’s utility extends to disease modeling, a key highlight of the study. In Alzheimer’s disease mouse models, the technique vividly revealed clusters of activated microglia surrounding beta-amyloid plaques, a hallmark of neurodegenerative pathology. This capability opens new avenues for exploring disease mechanisms, tracking pathological progression, and potentially evaluating therapeutic interventions at a cellular level within intact tissue samples.
Quantitatively, the signal amplification achievable through this enzymatic approach is striking. Compared to traditional fluorescent protein imaging or direct labeling with synthetic fluorophore-conjugated nanobodies, this method can enhance fluorescence intensity up to nine times at depths of 500 microns. Such an enhancement not only reduces imaging time but also improves detectability of low-abundance proteins, thereby facilitating high-throughput investigations and reducing resource consumption.
Despite its promising attributes, the authors candidly acknowledge certain limitations. Signal uniformity tends to diminish when imaging tissues thicker than 1 mm, and the enzymatic nature of signal amplification introduces non-linearity, complicating quantitative comparisons of antigen expression levels. Furthermore, the widespread application of this technique depends heavily on the growing availability of nanobodies specific to diverse targets, a resource currently limited but expected to expand rapidly as more nanobody sequences enter the public domain.
This innovative immunolabeling method represents a leap forward in volumetric imaging—overcoming persistent barriers that have historically constrained antibody penetration and signal sensitivity. The rapid, high-resolution, and versatile nature of POD-nAb/FT-GO 3D-IHC holds transformative potential not only for basic neuroscience research but also for clinical diagnostics and drug development. As demand surges for scalable and precise tissue imaging platforms, this technology emerges as a timely and powerful solution that could redefine standards in three-dimensional tissue analysis.
Both Dr. Yamauchi and Dr. Hioki envision a future where their method facilitates complex, multiscale mapping of neural circuits and pathological changes with cellular and subcellular precision. The accelerating pace of discoveries enabled by such tools promises to deepen our understanding of brain organization, neurodegenerative diseases, tumor microenvironments, and immune responses within tissue contexts.
With the ongoing refinement of nanobody libraries and the growing integration of cutting-edge tissue clearing and imaging technologies, POD-nAb/FT-GO 3D-IHC stands at the forefront of a new era in biological visualization. It exemplifies how clever biochemical engineering, combined with advanced microscopy, can unlock previously inaccessible vistas within intact biological specimens, propelling both fundamental research and clinical applications toward unprecedented horizons.
Subject of Research: Animal tissue samples
Article Title: A Three Dimensional Immunolabeling Method with Peroxidase-fused Nanobodies and Fluorochromized Tyramide-Glucose Oxidase Signal Amplification
News Publication Date: 18-Jun-2025
Web References:
https://doi.org/10.1038/s42003-025-08317-z
References:
Yamauchi, K., Koike, M., & Hioki, H. (2025). A Three Dimensional Immunolabeling Method with Peroxidase-fused Nanobodies and Fluorochromized Tyramide-Glucose Oxidase Signal Amplification. Communications Biology. https://doi.org/10.1038/s42003-025-08317-z
Image Credits:
Assistant Professor Kenta Yamauchi from Juntendo University, Japan
Keywords:
Histology, Immunohistochemistry, Immunohistochemical staining, Neuroscience