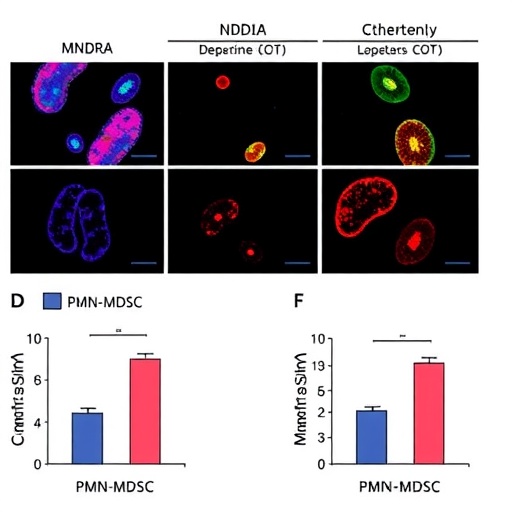
A groundbreaking study has emerged, shedding light on the complex interplay between immune regulation and colorectal cancer, particularly in cases characterized by high microsatellite instability (MSI-H). The authors, Zhang et al., have delved into the mechanisms by which the molecule known as MNDA (myeloid neoplasm down-regulated gene A) contributes to immunosuppression in this aggressive cancer subtype. Their research underscores how MNDA enhances the infiltration of polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) through the novel regulatory process of H3K18 lactylation, a modification that has not been widely explored in oncological contexts.
The significance of understanding the microsatellite instability-high variant of colorectal cancer cannot be overstated. This variant often indicates a specific genetic backdrop associated with high levels of mutation and a unique immune landscape. MSI-H colorectal cancers respond differently to treatments, particularly immune checkpoint inhibitors. Understanding the molecular mechanisms driving their behavior is crucial for developing more targeted therapies that can improve patient outcomes. The work presented by Zhang et al. expands our knowledge of this tumor type and introduces MNDA as a potential therapeutic target, revolutionizing how we approach MSI-H colorectal cancer.
As the authors unraveled the connection between MNDA and PMN-MDSC infiltration, they highlighted the role of H3K18 lactylation in this process. Histone lactylation is an epigenetic modification that fuels cancer progression and immune evasion. Prior to this study, histone modifications were largely understood in the context of DNA transcription regulation; however, this research positions lactylation as a key mediator of immune cell behavior. By modifying the chromatin landscape, H3K18 lactylation enables more favorable conditions for PMN-MDSCs to infiltrate tumor microenvironments, leading to significant immunosuppressive effects that enable cancer cells to survive and proliferate unchecked.
The implications of these findings are multi-fold. Firstly, the study raises a crucial question: how do we strategically inhibit the actions of MNDA or counteract its effects on PMN-MDSC infiltration? Targeting this pathway could mitigate the immunosuppressive environment that characterizes MSI-H colorectal cancer. Theoretically, inhibiting MNDA could promote the re-establishment of a more anti-tumor immune environment, paving the way for improved therapeutic responses—particularly with newer immunotherapy agents that aim to unleash the body’s immune system against tumors.
Developments in immunotherapy have already transformed the treatment landscape for various cancers, but MSI-H colorectal cancer remains challenging. Identifying the underlying mechanisms that foster cancer immune escape like those revealed in this study is vital for advancing treatment strategies. The interplay between MNDA and myeloid-derived suppressor cells draws attention not only to the tumor cells themselves but to their interactions with the surrounding immune cells, suggesting a more holistic approach to cancer treatment and prevention.
The research methodology employed by Zhang et al. is commendable, as the authors utilized sophisticated assays to dissect the molecular underpinnings governing the MNDA-PMN-MDSC relationship. The study included in vitro and in vivo experiments designed to elucidate how high levels of MNDA correlate with increased PMN-MDSC activity, supporting the hypothesis that MNDA functions as a facilitator of immunosuppression through epigenetic modification.
Incorporating patient-derived samples into their investigation, the researchers corroborated their findings with clinical relevance, establishing a connection between the laboratory results and real-world patient data. It’s exceptionally noteworthy how the integration of clinical samples enriches the translational potential of such studies, as it draws a clearer line from basic research to applicable therapies. This is precisely the type of research that holds promise in translating innovative findings into actionable strategies for clinical practice.
Additionally, the study opens up avenues for further research into the roles of various histone modifications in the tumor immunity landscape. Given the significance of H3K18 lactylation demonstrated in this work, subsequent studies may want to explore whether other lactylation marks play similar or complementary roles in immune response regulation. The potential for novel therapeutic interventions targeting histone modifications or MNDA presents a tantalizing frontier in cancer therapy.
Moreover, this study also brings various epigenetic changes into the conversation surrounding cancer treatment. As we have come to better understand the complexity of cancer as a disease driven by genetic and epigenetic factors, the role of modifications like H3K18 lactylation will likely become essential components in the future of precision oncology. A multi-targeted approach that encompasses not just genetic alterations but also epigenetic landscapes could yield meaningful improvements in the management of MSI-H colorectal cancer.
In the realm of colorectal cancer treatment, immunotherapy has shown promise, particularly as researchers refine strategies to exploit the unique immune response elicited by tumors. The unveiling of MNDA as a critical player in the immune-suppressive nature of MSI-H colorectal cancer could lead to the development of combination therapies, harmonizing traditional chemotherapy, newer targeted agents, and modulations of the immune response to provoke a stronger anti-cancer effect.
In conclusion, the research conducted by Zhang et al. is timely and vital in our quest to combat MSI-H colorectal cancer. By providing insights into the role of MNDA and PMN-MDSCs, the authors lay the groundwork for future explorations of innovative therapies designed to reverse immune suppression. Their work is expected to resonate deeply within the scientific community and encourage continued investigation into epigenetic mechanisms as potential therapeutic targets. Ultimately, such strides in our understanding of cancer biology hold hope for enhancing patient outcomes in the ever-evolving landscape of cancer treatment.
As the medical community digests these findings, the challenge remains: How can we effectively leverage this newfound knowledge to evolve treatment paradigms and craft regimens that meaningfully disrupt the immunosuppressive state of MSI-H colorectal cancers? The steps forward are filled with promise, urging researchers and clinicians alike to pursue these tantalizing possibilities with vigor and creativity.
Subject of Research: Immunosuppression in microsatellite instability-high colorectal cancer through MNDA and PMN-MDSC infiltration.
Article Title: MNDA promotes immunosuppression in microsatellite instability-high colorectal cancer by facilitating PMN-MDSC infiltration via H3K18 lactylation.
Article References:
Zhang, X., Wang, Wb., Cai, XY. et al. MNDA promotes immunosuppression in microsatellite instability-high colorectal cancer by facilitating PMN-MDSC infiltration via H3K18 lactylation.
J Transl Med 23, 1049 (2025). https://doi.org/10.1186/s12967-025-07097-8
Image Credits: AI Generated
DOI: 10.1186/s12967-025-07097-8
Keywords: MSI-H colorectal cancer, MNDA, PMN-MDSC, immunosuppression, H3K18 lactylation, epigenetics, immunotherapy.