A recent groundbreaking study has revealed the critical role of melatonin in protecting ovarian function from lipopolysaccharide (LPS)-induced damage. Conducted by researchers Shi, Ding, and Guo, the study highlights the detrimental impacts of LPS on ovarian health and elucidates how melatonin mediates protective effects through the JNK signaling pathway. This research not only enhances our understanding of ovarian physiology under stress conditions but indicates potential therapeutic applications of melatonin in reproductive medicine.
Ovarian health is paramount for female fertility and overall hormonal balance. The ovaries are responsible for the production of key hormones and the development of oocytes. Understanding factors that adversely affect ovarian health has been a focus of research, particularly regarding inflammatory responses that can be triggered by various agents, such as bacterial endotoxins. LPS, a component of the cell wall of Gram-negative bacteria, is known to induce inflammatory cascades that can critically impair ovarian function, leading to conditions such as ovarian dysregulation and infertility.
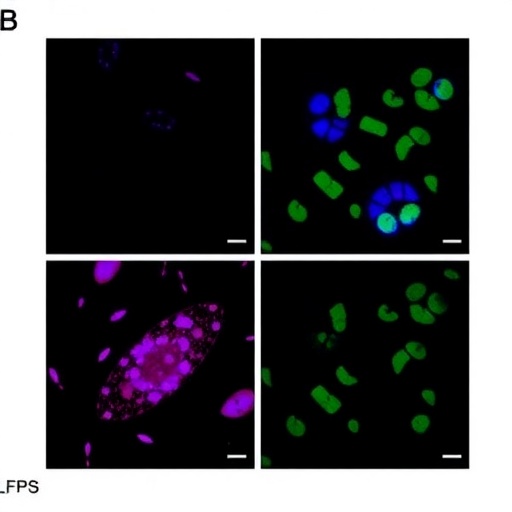
The study by Shi and colleagues investigates the protective mechanisms of melatonin, a hormone primarily produced by the pineal gland, against the oxidative stress and mitochondrial dysfunction induced by LPS. Melatonin is well-regarded for its antioxidant properties, contributing to its role in cellular defense against various forms of damage, including that which affects reproductive tissues. The researchers utilized a mouse model to simulate the effects of LPS on ovarian tissue, providing insights relevant to human health.
Research in this domain has consistently pointed to mitochondrial health as a crucial factor in ovarian function. Mitochondria, often referred to as the powerhouse of the cell, are intimately involved in energy production and the regulation of apoptosis. Mitochondrial dyshomeostasis—characterized by imbalances in mitochondrial function—can lead to cell death and dysfunction, impacting fertility. The current study emphasizes that when challenged with LPS, the ovarian tissue demonstrated significant mitochondrial impairment, highlighting the urgency of finding effective interventions.
Melatonin’s cellular signaling mechanisms have attracted major interest in recent years. The study identified that melatonin exerts its protective effects largely through the regulation of the JNK (c-Jun N-terminal kinase) signaling pathway. This pathway plays a pivotal role in responding to stress signals and mediating cellular survival and apoptosis. By modulating this pathway, melatonin can potentially restore balance in ovarian function even under inflammatory conditions, revealing a promising avenue for therapeutic application.
Experiments conducted in the study demonstrated that the administration of melatonin significantly attenuated the adverse effects of LPS on ovarian tissues. These findings indicate that melatonin not only helps to improve mitochondrial function but also assists in alleviating inflammation. The research paints a comprehensive picture of how melatonin acts on multiple fronts—from mitigating oxidative stress to improving the overall health of ovarian cells.
Moreover, the study meticulously assessed various biomarkers indicative of inflammation and oxidative stress, providing quantifiable evidence of the protective effects of melatonin. The results demonstrated reduced levels of pro-inflammatory cytokines and markers of oxidative damage in the treatment groups, strongly supporting melatonin’s role as a protective agent. The experimental evidence bolsters the hypothesis that melatonin could become a key player in reproductive health strategies.
The broader implications of these findings are immense, touching on various aspects of women’s health and potential interventions in reproductive disorders. As researchers delve deeper into the molecular actions of melatonin, there is an opportunity to explore its integration into clinical practices aimed at improving reproductive outcomes in women facing challenges related to inflammation and oxidative stress.
In summary, the study conducted by Shi and colleagues stands as a testament to the importance of melatonin in female reproductive health. By elucidating the mechanisms through which melatonin protects against LPS-induced damage, this research contributes significantly to the growing body of knowledge regarding the interplay between hormones, stress responses, and reproductive physiology. Given the increasing incidence of conditions that compromise fertility, such insights are not just academic; they hold real potential for developing novel therapeutic approaches.
As the excitement surrounding melatonin as a therapeutic agent for ovarian health continues to grow, future research will be critical. It will be important to determine dosing, safety, and efficacy in human populations, as well as to explore the broader implications of melatonin’s protective effects beyond just ovarian function. The road ahead may indeed lead to significant advancements in how we approach reproductive medicine and enhance the quality of life for those affected by reproductive health issues.
The study encourages researchers and clinicians to rethink traditional paradigms about ovarian impairment and inflammation. It opens up a dialogue about the underutilized potential of melatonin as a supplement in treatments aimed at improving not just ovarian health but overall reproductive wellness. Insight into these mechanisms will impact future research directions and clinical applications significantly.
In conclusion, this research on melatonin and its protective qualities against LPS-induced ovarian damage is a significant milestone in reproductive science. It illustrates the interplay of hormones and their capacity to mitigate damage in the context of inflammatory stressors. As the scientific community continues to investigate these complex interactions, the role of melatonin could emerge as a cornerstone in developing strategies to ensure optimal reproductive health for women worldwide.
Subject of Research: Melatonin’s protective role in ovarian damage due to LPS-induced mitochondrial dyshomeostasis.
Article Title: Melatonin Protects against LPS-Induced Mitochondrial Dyshomeostasis and Ovarian Damage through JNK Signaling Pathway in Mouse Ovary.
Article References:
Shi, LG., Ding, SM., Guo, TK. et al. Melatonin Protects against LPS-Induced Mitochondrial Dyshomeostasis and Ovarian Damage through JNK Signaling Pathway in Mouse Ovary.
Reprod. Sci. (2025). https://doi.org/10.1007/s43032-025-01954-z
Image Credits: AI Generated
DOI: 10.1007/s43032-025-01954-z
Keywords: Melatonin, ovarian health, LPS, JNK signaling pathway, mitochondrial function, inflammation, reproductive health.