Breast cancer continues to stand as one of the most formidable health challenges facing women globally. Despite advances in detection and treatment, the disease’s complexity demands deeper understanding, especially concerning the molecular and metabolic changes underpinning tumor growth and resistance. Recent scientific inquiry has turned a spotlight onto the metabolic reprogramming of breast cancer cells, uncovering how alterations in glucose, lipid, and amino acid metabolism collectively fuel malignancy and offer new therapeutic opportunities.
At the core of these metabolic shifts lies a well-documented phenomenon known as the Warburg effect. Unlike normal cells that rely predominantly on oxidative phosphorylation for energy, breast cancer cells preferentially utilize glycolysis for ATP production—even when oxygen is abundant. This reliance on aerobic glycolysis supports rapid energy turnover and provides intermediates for biosynthetic pathways critical for cell proliferation and survival. Detailed mechanistic studies reveal that this metabolic adaptation rewires key enzymes, transporters, and regulatory genes to maintain this energetic paradox, highlighting potential targets for disruption.
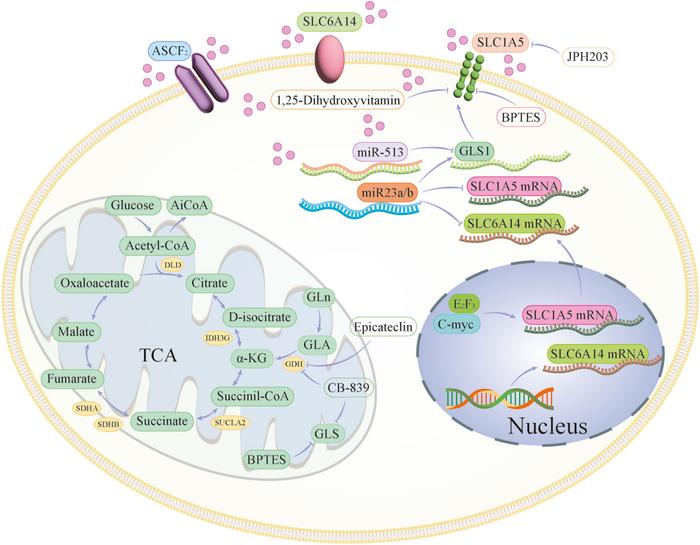
In addition to glucose metabolism, enigmatic changes in amino acid handling have emerged as pivotal for tumor sustenance. Glutamine, the most abundant amino acid in circulation, is extensively consumed by breast cancer cells to support nucleotide biosynthesis, redox balance, and anaplerosis within the tricarboxylic acid (TCA) cycle. The intricate interplay between glutamine metabolism and oncogenic signaling pathways orchestrates cellular proliferation and survival under metabolic stress. Current research is dissecting transporters and enzymes involved in glutamine uptake and catabolism, seeking to devise targeted inhibitors that can attenuate these metabolic dependencies and limit tumor growth.
Lipid metabolism represents another critical front in the metabolic landscape of breast cancer. Cancer cells not only enhance lipid synthesis to supply membrane biogenesis during rapid cell division but also engage lipid oxidation processes for supplemental energy. Beyond energy provision, lipid molecules participate in complex signaling cascades that influence metastasis, inflammatory responses, and resistance to pharmacological agents. Particularly in aggressive subtypes such as triple-negative breast cancer (TNBC), where limited targeted therapies exist, perturbations in lipid metabolic networks are increasingly recognized as drivers of malignancy and therapeutic resistance, opening novel avenues for clinical intervention.
The crosstalk between these diverse metabolic modalities underscores a nuanced network of adaptations cancer cells exploit for survival and growth. Recent multi-omics approaches integrating transcriptomics, metabolomics, and proteomics have revealed coordinated regulation of metabolic enzymes alongside oncogenic transcription factors, illustrating the plasticity of breast cancer metabolism. Such insights catapult the possibility of designing multi-targeted therapeutic regimens that simultaneously disrupt interconnected metabolic pathways, striving for improved efficacy and minimized resistance.
Despite the promising conceptual framework, translating metabolic insights into clinically viable treatments remains a formidable challenge. Several metabolic inhibitors are under preclinical and clinical investigation, yet their application is hampered by pharmacodynamic limitations, toxicity profiles, and heterogeneous patient responses. Tumor metabolic heterogeneity complicates uniform targeting, necessitating precision medicine approaches that incorporate metabolic phenotyping and biomarker-driven therapeutic selection.
An exciting frontier lies in integrating metabolic targeting with immunotherapy. Tumor metabolism profoundly influences immune cell function within the tumor microenvironment. Metabolic competition for nutrients like glucose and amino acids between cancer and immune cells can suppress antitumor immunity. By modulating metabolic pathways, researchers aim to rejuvenate immune effector functions and potentiate immunotherapeutic outcomes. This interdisciplinary convergence promises to redefine treatment paradigms, crafting personalized regimens that exploit metabolic vulnerabilities while enhancing the patient’s immune defenses.
On a molecular level, critical enzymes such as hexokinase 2 (HK2), glutaminase (GLS), and fatty acid synthase (FASN) have surfaced as central regulatory nodes in breast cancer’s metabolic network. Small molecule inhibitors and monoclonal antibodies targeting these enzymes are actively being explored. Emerging data underscore that combining metabolic inhibitors with conventional chemotherapy or targeted therapies may overcome resistance mechanisms and prevent disease relapse.
Moreover, the tumor microenvironment itself contributes to metabolic reprogramming by supplying alternative nutrients and metabolites, fostering a symbiotic relationship with cancer cells. Hypoxia, acidosis, and stromal cell interactions collectively modulate metabolic fluxes, further complicating the therapeutic landscape. Advances in imaging and metabolic flux analysis are illuminating these dynamic interactions, paving the way for more comprehensive treatment strategies.
The heterogeneity within breast cancer subtypes extends to their metabolic profiles. Hormone receptor-positive, HER2-enriched, and triple-negative tumors demonstrate distinct metabolic dependencies, which influence their responsiveness to metabolic interventions. Understanding these subtype-specific metabolic signatures can guide more tailored treatment regimens, improving clinical outcomes.
Impressively, the review also highlights advances in metabolic biomarkers that could serve as early indicators of breast cancer progression or therapeutic response. Metabolite profiling, integrated with genetic and epigenetic data, is enhancing diagnostic precision and enabling real-time monitoring of treatment efficacy.
The convergence of metabolic biology and oncology is reshaping our conception of breast cancer treatment. By unraveling the complex biochemical networks sustaining tumor cells, researchers are harnessing metabolism as both a diagnostic and therapeutic frontier. While challenges persist, particularly in balancing therapeutic efficacy with safety, the hope is that future clinical protocols will embody metabolic precision medicine—transforming breast cancer from a leading cause of mortality into a manageable condition.
The intricate metabolic reprogramming of breast cancer epitomizes the evolutionary ingenuity of cancer cells. In illuminating these pathways, science moves closer to unmasking vulnerabilities that can be exploited to halt tumor progression and improve survival. Multi-disciplinary efforts bridging molecular biology, pharmacology, and immunology hold the promise of ushering in a new era of therapies that are as sophisticated and adaptive as the disease they aim to conquer.
Subject of Research: Metabolic alterations and treatment strategies in breast cancer
Article Title: Landscape of metabolic alterations and treatment strategies in breast cancer
News Publication Date: 2025
References: Xiujuan Wu, Xuanni Tan, Yangqiu Bao, Wenting Yan, Yi Zhang, Landscape of metabolic alterations and treatment strategies in breast cancer, Genes & Diseases, Volume 12, Issue 5, 2025, 101521, DOI: 10.1016/j.gendis.2025.101521
Image Credits: Genes & Diseases
Keywords: Oncology, Breast cancer, Metabolic reprogramming, Warburg effect, Glutamine metabolism, Lipid metabolism, Triple-negative breast cancer, Precision medicine, Cancer metabolism, Immunotherapy