Recent advancements in neuroscience have shed light on the relationship between inflammation and neurodegenerative diseases, particularly in models of Parkinson’s disease. A notable study conducted by researchers, including Ngo, H.K.C., Srivastava, A., and Le, H., investigates the short-term effects of lipopolysaccharides (LPS) on astrocyte activation in LRRK2 G2019S knock-in mice. This study is particularly significant as it explores the intersection of immune response and neurodegeneration, aiming to enhance our understanding of Parkinson’s disease mechanisms.
Lipopolysaccharides, known as potent inflammatory agents, are recognized for their role in triggering immune responses. In this study, the authors subjected LRRK2 G2019S knock-in mice to a controlled short-term treatment with LPS. The primary objective was to determine whether this treatment would evoke a significant activation of astrocytes, the star-shaped glial cells in the brain that play critical roles in maintaining neural homeostasis and responding to injury.
Astrocyte activation is frequently observed in various neurodegenerative conditions, serving as a double-edged sword. While activated astrocytes can help protect neurons from damage, they can also contribute to neuroinflammation, potentially exacerbating neuronal injury. Hence, understanding the dynamics of astrocyte activation in the context of LPS exposure offers critical insights into the biological processes underpinning brain responses to pathological stimuli.
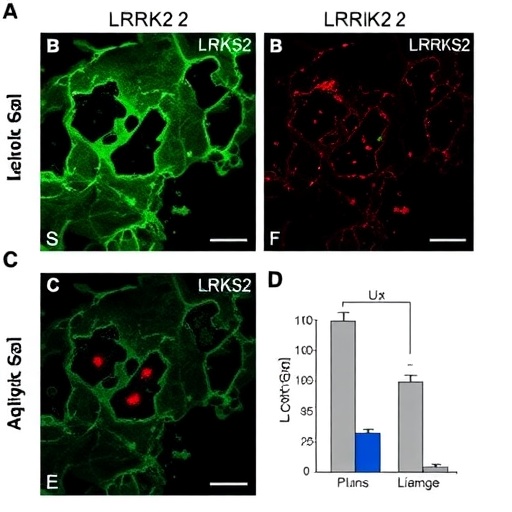
The researchers meticulously monitored the astrocytic responses following LPS administration. Using a combination of histological techniques and advanced imaging, they assessed changes in astrocyte morphology and expression of activation markers. The results were striking, as the treatment led to marked astrocyte activation without a correlative loss of dopaminergic neurons, a finding that challenges some established notions about neuroinflammatory responses in neurodegenerative disease contexts.
Given that the LRRK2 G2019S mutation is one of the most common genetic risk factors associated with familial and sporadic Parkinson’s disease, the insights drawn from this research may be particularly relevant for understanding the disease progression in affected individuals. These findings can lead to potential therapeutic avenues that target the inflammatory components involved in such diseases without compromising dopaminergic neuron integrity.
The research also reiterates the importance of a nuanced perspective on inflammation in neurological conditions. It aligns with emerging theories that advocate for a re-evaluation of the roles of various immune cells in the brain. By elucidating how astrocytes respond to inflammatory insults, this study hints at the necessity for therapies that can modulate astrocytic activity, potentially offering a dual benefit in protecting neuronal health while managing neuroinflammation.
Moreover, the absence of dopaminergic neuron loss post-LPS treatment highlights a pivotal area for future research. It raises intriguing questions about the resilience of dopaminergic neurons in the face of immune challenges and suggests that there may be protective mechanisms at play within the cerebral microenvironment that could be harnessed for therapeutic benefit. This could mark a significant paradigm shift in how we understand and approach the treatment of neurodegenerative diseases.
These findings underscore a critical need for further studies to dissect the molecular signals that underlie astrocyte activation and neuroprotection in the face of inflammatory stimuli. Identifying these pathways may not only advance our comprehension of neurobiology but could also catalyze the development of novel treatments aimed at mitigating the effects of neuroinflammation in diseases like Parkinson’s.
Overall, Ngo et al.’s work illustrates a vital aspect of the interplay between immune factors and neuronal health in the context of the LRRK2 G2019S mutation. As research continues to unveil the complexities of neuroinflammation, this study serves as a stepping stone towards understanding how these processes can be therapeutically modulated to preserve neuronal function and promote neurological health.
The implications of this research extend beyond basic neuroscience; they hold significance for public health strategies aimed at combating neurodegenerative diseases, which are increasingly prevalent in aging populations worldwide. The promising results open doors for interdisciplinary approaches, combining neurology with immunology to foster integrative strategies for treatment.
As the field progresses, it will be crucial to engage with these findings in a broader context, potentially reshaping our therapeutic conventions and research priorities in neurodegeneration. The study emphasizes the importance of continued exploration into how inflammatory processes affect brain health, urging scientists to consider both protective and detrimental aspects of immune responses.
In conclusion, the short-term lipopolysaccharide treatment reveals a fascinating dynamic within the neuroinflammatory landscape of LRRK2 G2019S knock-in mice. The research significantly enhances our grasp of astrocyte roles in response to inflammation while also indicating that protective mechanisms can exist alongside pathogenic processes. As we delve further into these intersections of immunity and neurodegeneration, we are likely to unearth transformative insights that could reshape the future of therapeutic approaches in the fight against diseases like Parkinson’s.
Subject of Research: The effects of short-term lipopolysaccharide treatment on astrocyte activation in LRRK2 G2019S knock-in mice.
Article Title: Short-term lipopolysaccharide treatment leads to astrocyte activation in LRRK2 G2019S knock-in mice without loss of dopaminergic neurons.
Article References: Ngo, H.K.C., Srivastava, A., Le, H. et al. Short-term lipopolysaccharide treatment leads to astrocyte activation in LRRK2 G2019S knock-in mice without loss of dopaminergic neurons. BMC Neurosci 26, 19 (2025). https://doi.org/10.1186/s12868-025-00939-7
Image Credits: AI Generated
DOI:
Keywords: Neuroinflammation, astrocytes, LRRK2 mutations, Parkinson’s disease, lipopolysaccharides, neurodegeneration, immune response, dopaminergic neurons, treatment strategies.