Cardiovascular disease remains a leading cause of mortality globally, with millions succumbing annually to its insidious effects. The human heart, despite its critical functions, possesses a remarkably limited ability to regenerate once damaged, presenting formidable challenges for medical science. This scenario raises an intriguing question: could we leverage the capability of our own cells to repair and regenerate heart tissue? Recent groundbreaking research conducted by a team led by Dr. Myeong-Hwa Song at Korea University has unveiled a promising innovative approach that may hold the key to transforming the field of regenerative medicine.
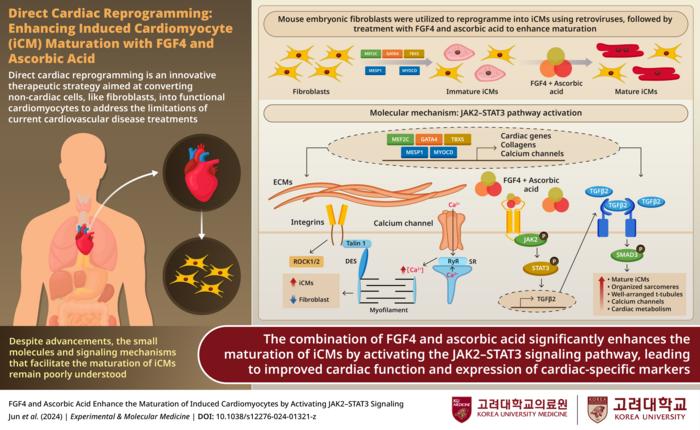
In a remarkable study, the researchers successfully converted fibroblasts, the most abundant connective tissue cells in the human body, into mature and functional induced cardiomyocytes (iCMs). This ambitious endeavor hinges on a unique combination of fibroblast growth factor 4 (FGF4) and vitamin C—a pairing that has been shown to accelerate cell maturation and enhance the functional capabilities of these newly generated cells. The implications of this research extend far beyond academic interest; they offer sincere hope for the future of patients suffering from heart disease.
Dr. Song remarks on the significance of their findings, emphasizing that this research is a pivotal step towards translating regenerative medicine into tangible therapies for patients. The method employed by the team utilizes direct cardiac reprogramming, enabling the transformation of fibroblasts into iCMs without passing through the stem cell stage. This route bypasses the complexities and risks associated with stem cell therapies, thereby providing a potentially safer and more efficient approach to heart tissue repair.
A significant barrier traditionally faced in this domain has been the challenge of generating fully functional, mature cardiomyocytes that can effectively mimic the native cells of the heart. The research team tackled this challenge by activating the JAK2–STAT3 signaling pathway, a crucial cellular mechanism responsible for various developmental processes essential for cell maturation. Their findings suggest that this activation plays a pivotal role in achieving improved structural configurations, which define the functionality of cardiomyocytes.
Utilizing a combination of advanced scientific techniques, including RNA sequencing, fluorescence imaging, and rigorous electrophysiological testing, the team observed not only improved cell structure but also enhanced electrical activity in the newly generated cardiomyocytes. The development of well-defined sarcomeres and T-tubules marked a notable advancement in the structural integrity of these cells, contributing to their overall functionality. Such refinements in cellular architecture indicate a step closer to engineering cells that operate in harmony with existing heart tissue, presenting an important milestone in regenerative medicine.
Additionally, the researchers noted improvements in ion channel function, further solidifying the electrical activity of the created cardiomyocytes. Enhanced electrical activity is crucial for the proper functioning of heart cells, which require efficient signaling for coordinated contractions. The discovery that inducing the JAK2-STAT3 pathway significantly boosts the maturation and functionality of iCMs offers extensive ramifications for therapeutic interventions, particularly in the domain of heart restoration after ischemic events like heart attacks.
The implications of such advancements in regenerative medicine are profound. Following the publication of their findings in the esteemed journal "Experimental & Molecular Medicine" on October 10, 2024, the excitement surrounding this work has the potential to spur further discoveries and advancements in cardiac repair strategies. The prospect of utilizing a patient’s own cells to engineer heart tissue offers the tantalizing possibility of reducing the need for donor organ transplants, which are fraught with challenges related to availability and compatibility.
Nevertheless, Dr. Song and his team are acutely aware that this is merely the genesis of a much broader journey. While the results are promising, extensive further studies are essential to ascertain the safety and efficacy of this novel approach within clinical contexts. As research progresses, the hope is to bridge the gap between laboratory achievements and real-world applications that can extend beyond traditional therapeutic boundaries.
In conclusion, the innovative techniques employed by Dr. Myeong-Hwa Song’s team at Korea University mark a significant advance in the pursuit of regenerative medicine. Their exploration into the conversion of fibroblasts into induced cardiomyocytes presents an encouraging frontier that may redefine treatment methodologies available for cardiovascular diseases. As the research landscape continues to evolve, the prospects of fully harnessing the intrinsic healing capabilities of the body could lead to transformative changes in how heart damage is treated, potentially saving countless lives affected by cardiovascular ailments.
This research not only reaffirms the excitement within the scientific community surrounding advancements in tissue regeneration but also underscores the necessity for ongoing studies that could bring this promising approach to clinical fruition. The future of cardiovascular therapy may very well depend on our ability to innovate and improve upon existing methodologies, paving the path for a new era in heart health and disease management.
Subject of Research: Cells
Article Title: FGF4 and ascorbic acid enhance the maturation of induced cardiomyocytes by activating JAK2–STAT3 signaling
News Publication Date: 1-Oct-2024
Web References: DOI: 10.1038/s12276-024-01321-z
References: Experimental & Molecular Medicine
Image Credits: The authors
Keywords
Regenerative medicine, Discovery research, Cardiomyocytes, Stem cell research, Cardiovascular disease, Clinical research, Fibroblasts, Electrophysiology, FGF pathway, Tissue repair, Cardiac function, Drug therapy, Growth factors, Vitamin C, Cardiac regeneration, Tissue regeneration, Stem cell therapy.