In a groundbreaking development in cancer treatment, researchers from the Korea Advanced Institute of Science and Technology (KAIST) have introduced an innovative approach aimed at transforming cancer cells into normal-like cells without the destruction typically associated with conventional therapies. This advanced methodology, led by Professor Kwang-Hyun Cho and his team from the Department of Bio and Brain Engineering, seeks to redefine the landscape of cancer therapies, enhancing the potential for targeted treatment with fewer side effects.
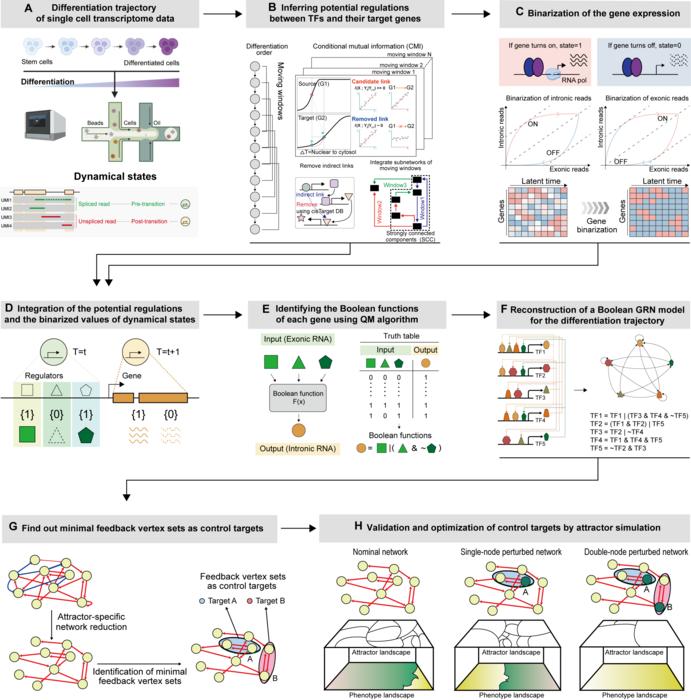
While traditional oncology primarily focuses on the eradication of tumors and cancer cells, this new technique involves reverting cancer cells to a more benign state. By harnessing the biological principles of cellular differentiation, the research team meticulously studied the differentiation trajectory of normal cells to establish a digital twin of the corresponding gene networks. This digital representation allows for an in-depth simulation of normal cell behavior and the identification of critical regulatory factors that govern transition along the differentiation pathway.
At the core of this research is the understanding that cancer cells often mimic states found in earlier stages of normal cell development. By observing the regression of normal cells during the process known as oncogenesis, the researchers identified master molecular switches responsible for controlling differentiation. Their findings suggest that inducing these switches in cancer cells enables a reprogramming effect, allowing their characteristics to resemble those of healthy cells.
The implications of this study are profound. Instead of annihilating cancer cells—which can lead to resistance and recurrence among patients—this innovative approach promotes the idea of ‘cancer cell reversion.’ The technique operates on the premise that through precise manipulation and systemic analysis, it might be possible to treat cancer without the detrimental collateral damage that often accompanies current interventions. By targeting the gene regulatory networks, the researchers can selectively influence the fate of cancerous cells, transforming them into a non-cancerous state.
To validate their findings, the research team employed an array of experimental methodologies, including both molecular and cellular experimentation, alongside animal studies. These comprehensive tests demonstrated that colon cancer cells could successfully revert back to a state akin to that of their healthy counterparts when exposed to the identified regulatory factors. This discovery not only showcases the potential effectiveness of cancer reversion as a therapeutic strategy but also introduces a new paradigm in understanding and addressing cancer biology.
Professor Cho articulated the significance of their findings, emphasizing the transformative potential of this approach in the realm of cancer treatment. The ability to convert malignant cells back to a normal phenotype signifies a major breakthrough that could change how oncology is practiced. He carefully noted that the systematic nature behind this reversion paves the way for developing therapies that could be more portable across various types of cancers, which is paramount in tailoring personalized treatments.
Furthermore, this study lays the groundwork for additional research in the field of cancer biology. The digital twin concept serves as a powerful tool in mapping cellular pathways and understanding the intricate dynamics of gene interactions. The ability to simulate these pathways opens avenues for exploring other metabolic or genetic disorders, potentially leading to new strategies in treating diverse diseases beyond just cancer.
The research has garnered substantial support, receiving backing from the Ministry of Science and ICT as well as the National Research Foundation of Korea. This support underscores the importance of the research and its potential to influence the biotechnology sector positively. Following the promising results, BioRevert Inc. will further develop these concepts into practical therapies designed to aid in the management and treatment of cancer, ushering in a new era of therapeutic options.
The implications of reversion therapies could ripple beyond clinical settings, with a potential impact on patient quality of life and treatment accessibility worldwide. As research in this domain continues to evolve, the dialogue surrounding cancer treatment modalities must shift toward integrative strategies that consider the biological complexities of cancer progression. These developments ignite hope for patients and oncologists alike, hinting at the possibilities of a future where cancer can be managed sustainably, without the dire consequences of traditional treatments.
Reflecting on the advancements made, it is crucial to recognize that the work of Professor Cho’s research team represents just the beginning of a future rich with potential. As more is understood about the regulatory mechanisms of cellular differentiation, the potential for developing targeted, less invasive treatment strategies appears limitless. This foundational work, published in the journal Advanced Science, not only contributes to the current body of knowledge but inspires further investigations into the realms of genetic engineering and therapeutic innovations.
Ultimately, this breakthrough in the treatment of colon cancer through the reversion of cancer cells to normal-like states marks a pivotal point in the fight against cancer and sets the stage for a new frontier in medical science, where the aim shifts from destruction to regeneration.
Subject of Research: Cellular differentiation pathways in cancer reversion
Article Title: Control of cellular differentiation trajectories for cancer reversion
News Publication Date: 11-Dec-2024
Web References: http://dx.doi.org/10.1002/advs.202402132
References: Advanced Science Journal, Wiley
Image Credits: KAIST Laboratory for Systems Biology and Bio-Inspired Engineering
Keywords: cancer treatment, cell reversion, differentiation trajectory, digital twin, gene networks, oncology, molecular switches, targeted therapy, KAIST, Professor Kwang-Hyun Cho.