A groundbreaking study published in the journal “Genes & Diseases” has introduced an innovative photosensitizer, Ce6-GFFY, designed specifically for the treatment of colorectal cancer, a malignancy known for its significant mortality rate. This research is pivotal, as it addresses the critical need for effective photosensitizers that can enhance the efficacy of photodynamic therapy (PDT). PDT is a cancer treatment modality that combines light-sensitive compounds and laser light to target and destroy cancer cells. However, many photosensitizers are not suitable for treating colorectal cancer, leaving a gap that this new compound aims to fill.
The Ce6-GFFY compound is a novel synthesis that ingeniously combines a self-assembling peptide known as GFFY with a recognized photosensitizer, chlorin e6 (Ce6). This combination not only exploits the inherent advantages of each component but also improves the properties of the resulting compound. Specifically, Ce6-GFFY self-assembles into macroparticles that measure approximately 160 nanometers in diameter. This size is significant because it enhances the compound’s bioavailability, enabling improved targeting of tumors. The modified structure also ensures a prolonged half-life of around 10 hours, making it an ideal candidate for sustained therapeutic action within the body.
Research revealed that Ce6-GFFY exhibits remarkable cellular penetration, allowing it to effectively reach and infiltrate cancer cells. Upon exposure to laser irradiation at 660 nm, this compound generates substantial quantities of reactive oxygen species (ROS). The formation of ROS is critical because these highly reactive molecules induce cellular stress and ultimately lead to cell death. This process, particularly in the context of colorectal cancer, triggers a cascade of immunological responses, effectively mobilizing the body’s immune system against tumors while also decreasing the presence of myeloid-derived suppressor cells—known for their role in tumor tolerance.
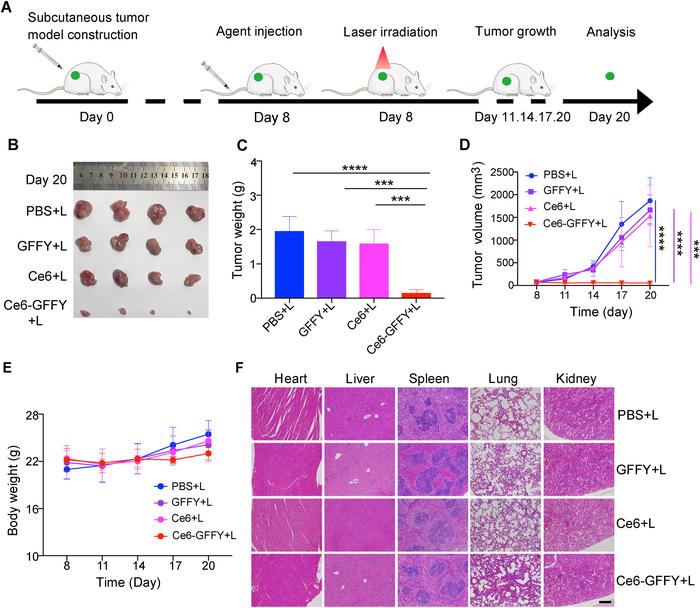
The study executed a series of in vivo experiments using CT26-derived subcutaneous tumor mice models to examine the efficacy of Ce6-GFFY. Initial injections of various agents, including PBS, GFFY alone, Ce6 alone, and a combined dose of Ce6-GFFY, were followed by systematic laser irradiation. Researchers noted significant differences in tumor responses based on the treatments administered. Notably, mice treated with Ce6-GFFY not only displayed reduced tumor mass but also a notable decrease in the growth rate of existing tumors, highlighting the therapy’s potential as a singular treatment modality.
Moreover, the safety profile of Ce6-GFFY appears exceptionally favorable. The study meticulously analyzed body weight changes in treated mice, a common indicator of toxicity in pharmacological studies. Results demonstrated minimal weight loss and no evidence of overt toxicity in the major organs examined, including the heart, liver, spleen, lungs, and kidneys. Pathological analyses using hematoxylin-eosin (H&E) staining further corroborated these findings, reinforcing the compound’s compatibility with normal tissue and underscoring its selective action against cancerous cells.
Given the robust mechanism behind Ce6-GFFY’s action and its significant in vivo efficacy, this research paves the way for future clinical applications. It addresses the unmet need for a reliable photosensitizer in colorectal cancer treatment, offering not just a means to target tumors, but also a way to engage the immune system in a war against cancer. Such approaches represent a paradigm shift in cancer treatment, moving towards therapies that can stimulate the body’s defenses rather than relying solely on direct cytotoxic actions.
The implications of Ce6-GFFY extend beyond colorectal cancer. The innovative mechanisms of action and the immunogenic effects stemming from this compound could inspire similar research endeavors for other types of cancer. By understanding and harnessing the interactions between engineered therapies and the immune system, researchers may establish a foundation for new treatment modalities across various malignancies.
As the scientific community continues to explore the full potential of Ce6-GFFY, it is evident that this study marks a significant step forward in the realm of oncological therapies. The dual action of direct tumor ablation combined with enhancing anti-tumor immunity positions Ce6-GFFY as a promising candidate in the fight against cancer—a foe that continues to challenge researchers and clinicians alike.
In conclusion, the introduction of Ce6-GFFY not only enriches the therapeutic arsenal against colorectal cancer but also exemplifies the innovative possibilities inherent in the convergence of chemistry, biology, and medicine. This research heralds a new era of precision medicine, where treatments can be tailored to engage specific biological pathways, thus enhancing the chances of successful disease management and patient recovery.
Subject of Research: Development of Ce6-GFFY as a novel photosensitizer for colorectal cancer therapy.
Article Title: Ce6-GFFY is a novel photosensitizer for colorectal cancer therapy.
News Publication Date: 2025
Web References:
References: Genes & Diseases, DOI: 10.1016/j.gendis.2024.101441.
Image Credits: Genes & Diseases.
Keywords: Anti-tumor immunity, Ce6-GFFY, Colorectal cancer, Novel photosensitizer, Photodynamic therapy