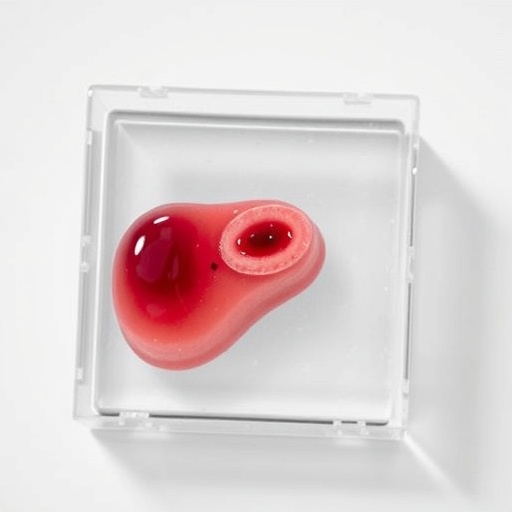
In a groundbreaking leap forward for cancer research and therapeutic development, scientists at the Terasaki Institute have engineered a revolutionary liver tumor-on-a-chip platform, meticulously designed to mimic the intricate vascular architecture and microenvironment of human liver cancers. This pioneering model, developed under the leadership of Dr. Vadim Jucaud, offers unprecedented insights into tumor biology and embolization therapy responses, heralding a new era in preclinical drug testing that promises greater predictive accuracy and ethical advancement.
Liver cancer remains a formidable global health challenge, with hepatocellular carcinoma (HCC) constituting the majority of cases. Traditional treatment modalities, including transarterial embolization—a technique that introduces occluding agents to artificially starve tumors—depend heavily on animal models for preclinical evaluation. However, interspecies differences in vascular structure, immune response, and cellular microenvironments often obfuscate translational relevance. The newly developed vascularized liver tumor-on-a-chip circumvents these limitations by incorporating a perfusable microvasculature within a three-dimensional tumor spheroid matrix, closely recapitulating the biophysical and biochemical conditions found in human liver tumors.
This microfluidic organ-on-a-chip device integrates tumor spheroids surrounded by engineered, capillary-like vessels capable of sustaining continuous perfusion and oxygen exchange. By simulating the hepatic artery’s physiological flow, the platform allows precise delivery and controlled occlusion of embolic agents directly within the vascular network. This feature replicates clinical embolization procedures with remarkable fidelity, enabling real-time observation of vascular remodeling, tumor cell viability, and angiogenic signaling pathways following treatment.
One of the core technical achievements of this model lies in its ability to quantitatively assess embolic agent efficacy through sophisticated readouts. These include high-resolution imaging of vascular regression, multiplexed cytokine profiling to understand inflammatory and immune dynamics, and surface marker expression analyses that elucidate cellular stress responses. Such multidimensional data acquisition surpasses traditional in vitro cell culture and in vivo animal studies, offering a dynamic, human-relevant window into the molecular cascades triggered by embolization therapies.
By advancing an ethically favorable alternative to animal testing, this platform aligns with the National Institutes of Health’s mission to promote the development and adoption of non-animal methodologies in biomedical research. The liver cancer-on-a-chip embodies this vision by enabling mechanistic studies in a controlled environment that faithfully mirrors human tumor microenvironments, thereby improving the predictive value of preclinical trials and accelerating the pipeline for novel therapeutic agents.
The implications for drug development extend beyond embolization therapies alone. This vascularized model acts as a versatile testbed for exploring synergistic treatment regimens, including chemoembolization and radioembolization, where therapeutic agents or radioactive beads are co-delivered with embolic materials. Understanding the nuanced interplay between these modalities and tumor vasculature at a cellular level promises to refine precision oncology approaches, tailoring interventions based on patient-specific vascular and tumor characteristics.
Beyond therapeutic evaluation, the liver tumor-on-a-chip offers profound insights into tumor biology, especially concerning hypoxia-induced signaling, immune cell infiltration, and angiogenesis – processes that are notoriously difficult to study in vivo due to their complexity and spatial heterogeneity. This model enables researchers to meticulously dissect these phenomena, increasing comprehension of tumor progression and resistance mechanisms, ultimately guiding the design of interventions that can disrupt the tumor microenvironment more effectively.
The technical sophistication of the microengineered vessels supports variable flow patterns and mechanical forces, facets critical to liver tumor vascular biology. This ability to simulate physiological shear stress and perfusion pressure fosters a microenvironment that sustains endothelial cell function and vessel integrity, elements essential for accurate modeling of drug delivery and embolization dynamics. Additionally, the platform’s modular design facilitates scalability and adaptability for high-throughput screening, offering substantial promise for industrial and academic research applications alike.
Dr. Huu Tuan Nguyen, first author of the seminal publication describing this platform, emphasizes the system’s transformative potential: by capturing the unique vascular dynamics responsible for hepatocellular carcinoma growth and therapeutic response, the on-chip model challenges the existing paradigm reliant on simplifications and cross-species extrapolations. This advancement enables researchers to probe cellular-level interactions under clinically relevant conditions, translating complex vascular reperfusion and occlusion phenomena into quantifiable outcomes.
Furthermore, the platform promotes a deeper understanding of embolization-induced alterations in tumor immune landscapes. Given that immune cell populations and cytokine networks significantly influence therapeutic efficacy and tumor recurrence, the ability to monitor these parameters longitudinally in a human-relevant model provides invaluable data that may inform future immunotherapies in conjunction with embolic treatments.
Notably, the Terasaki Institute’s liver tumor-on-a-chip spearheads an integrative approach that merges bioengineering, oncology, and immunology, reflecting the institute’s commitment to translating fundamental research into practical, impactful biomedical innovations. The development of such organotypic microfluidic systems epitomizes the future of personalized medicine by enabling the testing of patient-derived tumor samples under conditions that closely mirror in vivo physiology without the ethical and biological constraints inherent in animal models.
As the global scientific community continues to grapple with the limitations of traditional cancer models, the vascularized embolization-on-a-chip represents a landmark achievement, setting a new standard for preclinical evaluation and offering hope for faster, safer translation of experimental therapies into the clinic. This advancement may profoundly influence not only liver cancer treatment paradigms but also broader applications across vascularized tumor types.
With publication in the journal Biofabrication in August 2025, this research lays a foundational platform that invites further exploration and collaborative innovation. The Terasaki Institute’s multifaceted approach to microfluidic system design, coupled with precise biological validation, signals a transformative shift in how researchers can emulate human disease conditions, study complex pathophysiology, and develop next-generation therapeutics with improved clinical relevance.
Contact with the principal investigator, Dr. Vadim Jucaud, is encouraged for those seeking to collaborate or learn more about this cutting-edge technology. As biomedical innovation continues to accelerate, models such as this will be indispensable tools in the pursuit of effective, patient-tailored cancer therapies that harmonize scientific rigor with ethical responsibility.
Subject of Research: Cells
Article Title: Embolization-on-a-chip: Novel Vascularized Liver Tumor Model for Evaluation of Cellular and Cytokine Response to Embolic Agents
News Publication Date: 3 September 2025
Web References: http://dx.doi.org/10.1088/1758-5090/adfbc3
References:
Jucaud, V., Nguyen, H. T., Peirsman, A., Khorsandi, D., Dokmeci, M. R. (2025). Embolization-on-a-chip: Novel Vascularized Liver Tumor Model for Evaluation of Cellular and Cytokine Response to Embolic Agents. Biofabrication. DOI: 10.1088/1758-5090/adfbc3
Image Credits: Terasaki Institute
Keywords: Cancer, Liver cancer, Biomedical engineering, Tissue engineering, Drug delivery