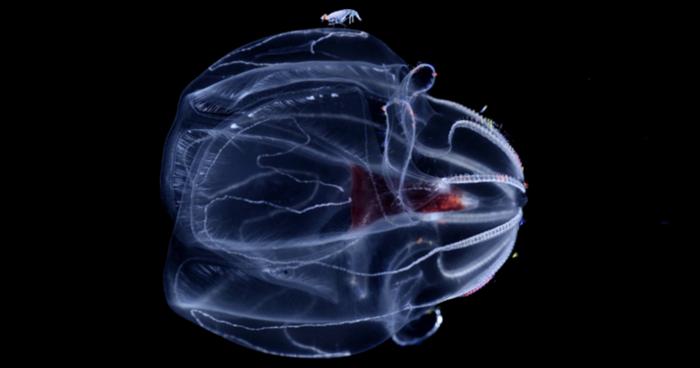
A multi-institutional team that includes researchers from the University of Delaware, University of California San Diego and Monterey Bay Aquarium Research Institute (MBARI), among others, published a paper in Science on Thursday, June 27, that provides new insight on how deep-sea “comb jellies” called ctenophores adapt and survive at extreme pressures.

Credit: Courtesy of the Lyman group and Jacob Winnikoff/UC San Diego
A multi-institutional team that includes researchers from the University of Delaware, University of California San Diego and Monterey Bay Aquarium Research Institute (MBARI), among others, published a paper in Science on Thursday, June 27, that provides new insight on how deep-sea “comb jellies” called ctenophores adapt and survive at extreme pressures.
It turns out that part of the adaptation involves lipids, fatty chemical compounds found in the membrane of all living cells that perform essential functions, including storing energy, sending signals and controlling what passes through the cell membrane.
The work provides new knowledge about how marine organisms can adapt and survive in the ocean, now and potentially into the future. It also may inform what’s known about the human body — in particular, how a specific lipid called plasmalogen found in nerve cells might work in our brains.
UD biophysicist Edward Lyman and doctoral students Sasiri Vargas-Urbano and Miguel Pedraza Joya are among the co-authors on the paper. Other co-authors include first-author Jacob Winnikoff, a researcher at MBARI and University of California, Santa Cruz and San Diego, now at Harvard University, Steven Haddock, an MBARI marine biologist, and the project principal investigator Itay Budin, an assistant professor of chemistry and biochemistry at UC San Diego. Additional collaborators include researchers from UCSD Health Sciences, University of California Santa Cruz, the National Institutes of Health and Cornell Center for High Energy X-ray Sciences.
Adapting under extreme pressure
Ctenophores are predators found at various depths in the ocean, where they help regulate the marine ecosystem by eating fish and shellfish larvae, while serving as a food source for other marine animals. If you go back in time, UD’s Lyman said, the first thing that branches off from the rest of the animals is the ctenophores — a result just established last year by co-author Haddock.
“This means that you and I are more closely related to a jellyfish than a jellyfish is to a ctenophore,” Lyman said.
The deep ocean, meanwhile, is characterized by low temperature and high pressure. Lyman explained that ctenophores are great for teasing apart this problem of how marine organisms adapt to extreme pressure environments because there are multiple ctenophore species that live at the surface, up to two and half miles deep in the ocean, and only at the surface in the Arctic, where the temperature is generally the same as in the deep sea.
“Studying ctenophores, you can compare those organisms in a way that’s roughly controlled for temperature and now you can look at how the organism adapts only to changes in pressure,” said Lyman, a UD professor of physics and astronomy with expertise using molecular dynamics simulations to characterize lipids.
Haddock’s team at MBARI collected samples of 17 different species of ctenophores that live in different parts of the ocean and Budin’s lab analyzed their lipidomes — the chemical species found in the cell membrane — in an extensive survey.
In the work, the collaborative researchers compared the chemical composition of shallow- and deep-sea dwelling ctenophores and found an adaptation in the cellular membrane of those living in the deep that enables them to survive under extreme pressures. Budin’s preliminary research revealed that deep-sea ctenophores have a huge abundance of a particular type of lipid molecule called plasmalogen, which is present in our own membranes in small amounts.
“What makes plasmalogen lipids interesting is that they allow cell membranes to bend and deform, even in the deep ocean at high pressure, where membranes would otherwise be very stiff, and that’s a useful adaptation,” said Lyman.
Of molecules and membranes
A fundamental function of membranes is to let things in and out a cell or to enable cell division to replicate genetic material. To do this, the cell membrane must go from a generally flat state to one that is highly curved, so the researchers ran experiments and simulations to measure the shape of plasmalogen molecules under different conditions. This is because while all membranes are made from a mixture of different kinds of lipids, it’s the chemistry of a lipid molecule that determines whether it wants to reside in a membrane that’s flat or curved.
UD’s Vargas-Urbano leveraged molecular dynamics and the massive computational power of National Science Foundation supercomputers to model all the structures inside the ctenophore membranes and simulate how the molecules would move and interact with each other at high pressures. The process took a great deal of time.
“Simulations that are about 500 nanoseconds long could take about a month to create from the data,” said Vargas-Urbano. That’s the equivalent of a movie clip that lasts about 500 billionths of a second.
Meanwhile, Budin and Winnikoff used a special X-ray scattering beam line at Cornell to experimentally study how the structure of ctenophore membranes changed under various pressures. Looking at the structural properties of the lipid mixtures directly from the organisms at high-pressure was a crucial part of the project that helped the researchers discern that ctenophore lipidomes are specialized for high pressure.
The deep sea is under extreme pressures equal to that of hundreds of atmospheres, due to the weight of the water that lies above. Budin’s team at UC San Diego learned that if they exposed the cell membrane of E. coli, a human gut bacteria, to pressures found where the deepest ctenophore live (roughly 500 times the water pressure found on the ocean surface) then the microbes’ growth was severely restricted, Lyman said. However, when the researchers gave E. coli the ability to synthesize plasmalogen lipids and pressurized them the same way, then the cells could grow and divide normally.
Simulating the membrane in the computer and testing it under various temperatures and pressures across various timeframes allowed the UD team to validate that it is the plasmalogen lipids that keep the membranes fluid and deformable at high pressure.
“Using molecular-dynamic simulations to explore this system, we were able to test conditions that would be found even deeper in the sea than these ctenophore species actually live to see what happened,” said Vargas-Urbano.
This is because the deep-sea ctenophores contained more plasmalogen lipids in their cell membranes than other ctenophore species found at the sea surface and in the Arctic. In particular, the deep-dwelling ctenophores had higher concentrations of a plasmalogen known as PPE, which is characterized by a distinct cone shape. Simulations by the UD research team and artificial membrane experiments by Budin and Winnikoff showed the more PPE plasmalogen was present, the more the membranes curled up, even at low pressures.
One surprising result from the research, Lyman said, is that when they plotted the 17 ctenophore species studied, the researchers discovered a significant correlation between how much plasmalogen is found in the species’ cellular membrane and where it can live.
“If you take a deep-sea ctenophore and bring it to the surface, its membrane bends like crazy and that’s no good. If you take a surface-dwelling ctenophore and bring it to the deep, its membrane won’t bend anymore, also not good,” Lyman said.
But when the researchers compared the membrane of ctenophores living in surface waters and their deep-sea cousins in their natural environments, the species had similar properties in terms of how the molecules inside the cells adjust to keep the membrane stable.
The researchers called this effect “homeocurvature adaptation,” because the ctenophores—and their lipidomes—had adapted to the situation that they’re living in. This understanding may help explain a long-standing mystery of why deep-sea invertebrates, including ctenophores, disintegrate at the surface no matter how carefully they are handled. It seems in some cases the membranes actually are held together by the extreme pressure.
Human health implications of the work
Knowing how life adapts to high pressure and extreme environments such as the ocean can also inform what is known about the human body. Lyman explained that plasmalogen lipids present in ctenophores also are found in our bodies, most abundantly in neural tissue.
“Nerve cells in the brain transmit messages by sending chemicals from one cell to another. And there is an awful lot of plasmalogen at the site where all that synaptic transmission is happening in your neurons,” said Lyman.
The loss of plasmalogen is known to be associated with conditions such as Alzheimer’s disease, making new insights about the unique properties of plasmalogen lipids potentially useful in other areas of research.
Full list of authors: Daniel Milshteyn, Edward Dennis, Aaron Armando, Oswald Quehenberger and Itay Budin (all UC San Diego); Jacob Winnikoff (Harvard University); Sasiri J. Vargas-Urbano, Miguel Pedraza Joya and Edward Lyman (all University of Delaware); Alexander Sodt (National Institute of Child Health and Human Development); Richard E. Gillilan (Cornell University); and Steven H.D. Haddock (MBARI).
Journal
Science