Chronic inflammatory bowel disease (IBD), encompassing conditions such as ulcerative colitis and Crohn’s disease, represents a profound clinical challenge with serious implications including an elevated risk of colorectal cancer. These debilitating ailments primarily affect young adults between the ages of 15 and 29, a critical period that intersects crucial educational and vocational development stages. Despite advances in treatment modalities aimed at symptom control and immunosuppression, relapse and progression remain prevalent. Now, a breakthrough discovery by researchers at Charité – Universitätsmedizin Berlin sheds light on a specific immune pathway that could pave the way for more precise, effective therapeutic interventions against chronic intestinal inflammation and its malignant transformation.
IBD is characterized by recurring episodes of inflammation within the gastrointestinal tract, eliciting symptoms such as severe abdominal pain, diarrhea, fatigue, and weight loss. Ulcerative colitis restricts inflammation primarily to the colonic mucosa, whereas Crohn’s disease can affect any layer of the gastrointestinal wall and any part of the digestive tract, from mouth to anus. The persistent inflammatory milieu gradually damages the mucosal barrier and deeper tissue layers, increasing not only patient morbidity but also lifetime risk for colorectal carcinoma. Traditional therapies largely rely on broad immunosuppression, which while mitigating symptoms can sometimes compromise systemic immunity, underscoring a critical need for targeted interventions.
In an extensive research effort led by Prof. Ahmed Hegazy at Charité’s Department of Gastroenterology, Infectiology and Rheumatology, the molecular underpinnings driving chronic inflammation in IBD have been delineated with unprecedented clarity. The team identified a deleterious interaction between two immune messengers: Interleukin-22 (IL-22) and oncostatin M (OSM). While IL-22 generally serves a protective function by sustaining the intestinal epithelial barrier integrity and promoting tissue repair, it paradoxically also primes the gut lining for heightened responsiveness to oncostatin M by increasing the abundance of OSM receptors on gut cells. This synergistic interplay precipitates an uncontrolled inflammatory cascade.
The inflammatory signaling orchestrated by OSM, a cytokine produced by activated immune cells, initiates and perpetuates a hyperactive immune environment by triggering downstream inflammatory agents. Interestingly, the research revealed that patients with elevated OSM expression levels exhibited resistance to established IBD treatments, suggesting that OSM could serve as a prognostic biomarker for identifying individuals at risk of therapeutic non-responsiveness. This insight holds immense clinical potential in guiding personalized medicine approaches for IBD management.
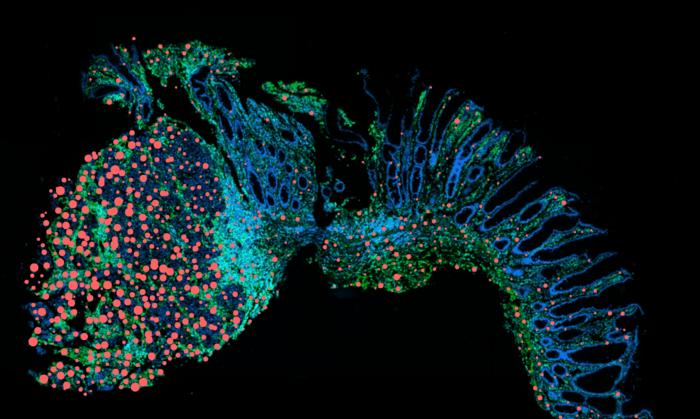
Utilizing sophisticated single-cell RNA sequencing technologies, Hegazy’s team catalogued the cellular composition and receptor expression profiles within inflamed intestinal tissues in both animal models and patient biopsies. These analyses uncovered a conspicuous enrichment of diverse cell populations exhibiting heightened OSM receptor density in inflamed gut areas compared to healthy counterparts. Furthermore, this OSM receptor upregulation was markedly amplified where IL-22 signaling was elevated, confirming the mutually reinforcing nature of these cytokines in driving chronic gut inflammation.
To directly explore potential therapeutic avenues, experimental blockade of OSM receptor activity was employed in preclinical models. Remarkably, inhibiting this receptor-ligand interaction significantly attenuated intestinal inflammation and reduced the incidence and progression of colorectal tumors arising in the context of chronic inflammation. These findings illuminate the critical role that the IL-22/OSM axis plays not only in perpetuating immune dysregulation but also in facilitating tumor-promoting microenvironments.
Importantly, the researchers identified a selective accumulation of OSM receptor–positive cells in tumor-adjacent tissues from colorectal cancer patients with a history of chronic intestinal inflammation, a pattern not observed in non-inflamed healthy tissues. This spatial localization underscores the hypothesis that the IL-22/OSM axis contributes to oncogenic processes, likely by sustaining a pro-inflammatory and tissue-remodeling milieu conducive to malignant transformation.
Dr. Britta Siegmund, Director of the Clinic for Gastroenterology at Charité, emphasized the heterogeneity and complexity of chronic inflammatory bowel diseases across patients, noting that variable cytokine profiles and immune cell interactions complicate therapeutic predictability. The discovery of the IL-22–oncostatin M interplay provides a vital mechanistic framework to classify disease subtypes and stratify patients more accurately based on their underlying pathophysiology and therapeutic responsiveness.
Capitalizing on these translational insights, a clinical trial is already underway to evaluate an antibody targeting the OSM receptor, aiming to disrupt this pathogenic signaling and achieve remission in severely affected IBD patients. This targeted approach marks a departure from broad-spectrum immunosuppression by directly neutralizing a critical inflammation amplifier, potentially reducing side effects and improving efficacy.
The study underscores the vital importance of precision immunology in tackling chronic inflammatory diseases and their sequelae. By elucidating the molecular crosstalk between IL-22 and oncostatin M, these findings herald a promising new era in the management of IBD, offering hope for more durable disease control and prevention of associated bowel cancer.
Financial support for this landmark work was provided by prominent institutions including the European Research Council (ERC), the German Research Foundation (DFG), and the Volkswagen Foundation. Collaborative efforts involving scientists from the German Rheumatism Research Center and industry partners such as Genentech further exemplify the critical synergy of multidisciplinary approaches in driving innovative therapeutic development.
This discovery not only advances our fundamental understanding of immune dysregulation in chronic intestinal diseases but also highlights the complex balancing act within the immune system, where protective mechanisms like IL-22 can, under certain pathological conditions, become complicit in damaging inflammation through their interaction with OSM. Future research may also explore how modulation of this axis impacts the broader systemic immune response and tumor microenvironment interactions.
As the global burden of IBD continues to rise, innovations such as targeting the IL-22–oncostatin M axis illuminate a path forward toward personalized medicine and improved patient outcomes. The convergence of cutting-edge single-cell analytics, targeted molecular therapies, and integrated clinical research heralds a transformative moment in combating this multifaceted disease.
Subject of Research: People
Article Title: The IL-22–oncostatin M axis promotes intestinal inflammation and tumorigenesis
News Publication Date: 07 November 2024
Web References:
- Original publication: https://www.nature.com/articles/s41590-025-02149-z
- Department of Gastroenterology, Infectious Diseases and Rheumatology: https://gastro.charite.de/en/
- AG Hegazy "Inflammatory mechanisms": https://gastro.charite.de/en/research/rg_hegazy
- Press release: https://www.charite.de/en/service/press_reports/artikel/detail/unlocking_predictors_of_success_in_treating_inflammatory_bowel_disease_ibd
References:
Cineus R, et.al. The interleukin 22-oncostatin M axis promotes intestinal inflammation and tumorgenesis. Nature Immunology. 2025 May 30. doi: 10.1038/s41590-025-02149-z
Image Credits: © Charité | Ahmed Hegazy
Keywords:
Inflammatory bowel disease, ulcerative colitis, Crohn’s disease, oncostatin M, interleukin-22, chronic inflammation, cytokines, colorectal cancer, targeted therapy, immune signaling, biomarker, intestinal tumorigenesis