In a groundbreaking study published in Journal of Ovarian Research, researchers have unveiled crucial insights into the role of hnRNPA2B1 in regulating granulosa cell ferroptosis, a form of regulated cell death that has been implicated in premature ovarian failure (POF). This condition is characterized by the loss of ovarian function in women under the age of 40, and the underlying mechanisms remain poorly understood. The latest findings shed light on the potential molecular pathways that govern cell survival within ovarian tissues, which may ultimately lead to innovative therapeutic avenues for addressing this significant reproductive health issue.
Ferroptosis, distinct from traditional forms of apoptosis, is initiated by lipid peroxidation and characterized by an iron-dependent accumulation of reactive oxygen species. This process has garnered considerable attention in various fields of biological research, yet its implications in ovarian biology have been largely uncharted territory until now. Specifically, this study highlights the pivotal role of granules and other specialized cellular structures in the execution of ferroptosis, adding complexity to the understanding of cellular fate in ovarian health.
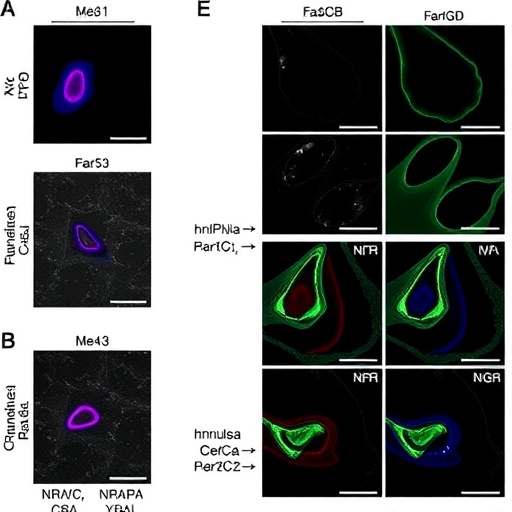
At the core of this research is the multifaceted protein hnRNPA2B1, known primarily for its involvement in RNA metabolism. Researchers have long speculated about the potential of hnRNPA2B1 to influence various cell signaling pathways, but its role in ferroptosis presents a novel angle. By mediating the m^6A modification of RNA—specifically, through the modulation of SLC7A11, which encodes a cystine/glutamate antiporter—hnRNPA2B1 appears to act as a crucial gatekeeper in cell survival, presenting a fine-tuned regulatory mechanism that could tip the balance between life and death in granulosa cells.
This revelation regarding the m^6A/SLC7A11 axis has far-reaching implications. SLC7A11 is integral to maintaining cellular redox homeostasis and regulating oxidative stress, elements that are critical for normal ovarian function. Dysregulation in this pathway, particularly through alterations in hnRNPA2B1 expression or activity, could predispose granulosa cells to ferroptosis, thereby contributing to POF. Understanding this relationship invites further exploration into how these molecular events intersect with environmental factors and genetic predispositions that lead to premature ovarian failure.
The experimental framework of the study involved a combination of in vitro assays and advanced molecular techniques, including CRISPR-Cas9 gene editing to delineate the functional roles of hnRNPA2B1 and SLC7A11. Data revealed that silencing hnRNPA2B1 led to increased ferroptosis in granulosa cells under stress conditions, suggesting a protective role against oxidative damage. The analytical methodologies employed provide a robust validation of the biological significance of hnRNPA2B1 and its downstream effects, creating a solid foundation for future research avenues.
Additionally, the team utilized transcriptomic and proteomic analyses to gather comprehensive insights into the cellular responses triggered by the manipulation of hnRNPA2B1 expression levels. Their findings revealed noteworthy changes in the expression of genes that are pivotal in regulating oxidative stress responses and lipid metabolism, highlighting the multifaceted impact of hnRNPA2B1 on cellular homeostasis. This data further cements the protein’s role as a crucial player in maintaining granulosa cell viability amid challenging conditions.
The implication of these findings extends beyond POF; they touch upon broader themes in ovarian biology and women’s health. As the understanding of ferroptosis expands, there is potential for the development of targeted therapies aimed at modulating this process, thereby offering hope to women facing reproductive challenges due to premature ovarian failure. By elucidating the protective mechanisms afforded by hnRNPA2B1, researchers aim to pave pathways for novel interventions resourcing ovarian function restoration.
Moreover, the research raises intriguing questions regarding the interplay of global health and ongoing reproductive challenges faced by women in various contexts. As increasing numbers of women delay childbearing, the urgency for effective strategies to combat infertility emphasizes the need for continued investigation into fundamental reproductive biology, particularly as it relates to emerging cellular death pathways like ferroptosis.
A significant hallmark of this study is the fusion of basic biological research with potential clinical application. By revealing the complexity of signals that govern granulosa cell survival, the authors invite both external validation and the scientific community’s engagement in unraveling therapeutic strategies that may eventually mitigate the impacts of POF. Building on these findings, future studies could focus on exploring the therapeutic modulation of hnRNPA2B1 and its associated pathways, seeking to develop adjunct therapies that protect ovarian function in vulnerable populations.
In summary, the study, which offers a fresh perspective on the molecular pathways influencing ovarian health, places hnRNPA2B1 firmly at the center of a new narrative in reproductive biology. Its emerging role in the regulation of ferroptosis underscores the intricate balance that sustains cellular health within the ovaries and highlights the potential for future therapeutic advances aimed at preserving fertility in women experiencing premature ovarian failure.
The findings presented evoke a compelling sense of urgency and significance, as they illuminate not only a critical biological process but also the societal implications stemming from women’s reproductive health challenges. The integration of cutting-edge molecular techniques with insightful biological inquiry provides a roadmap for future explorations that could redefine our understanding of ovarian function and the factors leading to infertility.
As the scientific community digests these transformative insights, the call for collaboration among researchers, clinicians, and advocates for women’s reproductive health becomes ever more compelling. Through concerted efforts, we may yet unravel the complexities of ovarian biology, leading toward strategic interventions that safeguard the fertility and well-being of future generations.
The implications of this research extend not only to scientific inquiry but also impact women’s health practices and policy discussions surrounding reproductive rights and health access. As we continue to explore the connections between molecular mechanisms and their physiological manifestations, the promise of new treatments and the safeguarding of reproductive integrity remains at the forefront of scientific inquiry.
In a world where reproductive health is increasingly recognized as a cornerstone of overall well-being, this study serves as a clarion call for further research on the mechanisms underpinning fertility and the critical importance of understanding women’s health in broader biomedical contexts.
With the groundwork laid by this study, myriad possibilities unfold, beckoning further inquiry into the molecular rulers of fertility, advocacy for women’s health, and ultimately, the empowerment of women to understand and manage their reproductive health throughout their life trajectories.
Subject of Research: Granulosa cell ferroptosis and its regulation by hnRNPA2B1 in premature ovarian failure.
Article Title: hnRNPA2B1 restrains granulosa cell ferroptosis by m^6A/SLC7A11 in premature ovarian failure.
Article References:
Xiong, J., He, L., Zhang, Y. et al. hnRNPA2B1 restrains granulosa cell ferroptosis by m6A/SLC7A11 in premature ovarian failure.
J Ovarian Res 18, 165 (2025). https://doi.org/10.1186/s13048-025-01718-y
Image Credits: AI Generated
DOI:
Keywords: Granulosa cells, ferroptosis, hnRNPA2B1, premature ovarian failure, reproductive health, fertility, molecular mechanisms.