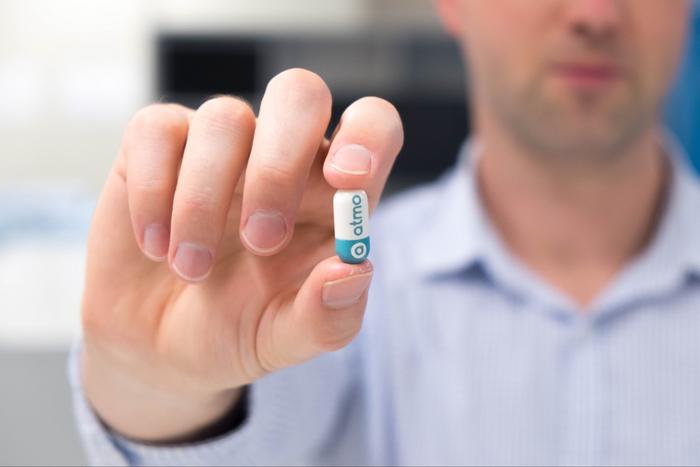
In a groundbreaking stride toward transforming gastrointestinal health diagnostics, an ingestible gas-sensing capsule known as the Atmo Gas Capsule has advanced significantly, moving closer to widespread clinical use. Developed initially at RMIT University, the innovative device has had its intellectual property rights fully transferred to medical technology company Atmo Biosciences. This pivotal transition not only amplifies Atmo’s capability to drive regulatory approval and commercialization but also reinforces a strategic partnership that promises to revolutionize how gut health is understood and managed.
The Atmo Gas Capsule is designed to measure gaseous biomarkers directly within the gastrointestinal tract, providing unprecedented real-time insights into the complex environment of the human gut. Traditional diagnostic methods typically rely on indirect assessments or invasive procedures that offer limited spatial or temporal resolution of gut activity. In contrast, this ingestible technology traverses the digestive system, detecting and quantifying gut gases at their precise origin points. These measurements help evaluate key physiological parameters such as gut transit time, a critical marker for diagnosing prevalent motility disorders, including gastroparesis and slow transit constipation.
Functional gastrointestinal disorders impact approximately 40% of the global population, yet remain notoriously difficult to diagnose accurately due to the episodic and multifaceted nature of symptoms. The Atmo Gas Capsule addresses this challenge by providing continuous, localized, and objective data on intestinal gas production and movement dynamics. By capturing the concentrations of gases like hydrogen and methane, which are metabolites produced by gut microbiota during digestion, clinicians and researchers can better understand disruptions in gut motility and microbiome interactions that contribute to disease pathology.
Originally licensed to Atmo Biosciences in 2018 following pioneering research at RMIT, the technology has undergone rigorous refinement and validation. Over the ensuing years, Atmo has successfully transitioned the capsule from an early-stage concept to a sophisticated, clinically viable device. This progression entailed the development of advanced manufacturing processes, miniaturized sensor arrays, and reliable wireless communication protocols, enabling data transmission from within the gut to external monitoring systems. Each step has been essential to ensuring that the capsule operates safely and effectively within the challenging environment of the digestive tract.
A recent landmark clinical trial involving over 200 participants across 12 sites in the United States and Australia underlined the device’s safety and accuracy. This pivotal study confirmed that the Atmo Gas Capsule consistently captures meaningful biosignatures of gastrointestinal function without adverse events, positioning it favorably for regulatory evaluation. The device is currently undergoing review by the U.S. Food and Drug Administration (FDA) under the 510(k) pathway. Upon clearance, it is expected to offer a profoundly more informative alternative to conventional diagnostic tools used in gastroenterology.
Importantly, the utility of the Atmo Gas Capsule extends beyond traditional diagnostic applications. Collaborative trials, such as one conducted at Florida State University, have explored its capacity to monitor the physiological impact of dietary interventions on gut gases and transit kinetics. Such research illustrates the capsule’s potential to contribute crucial data for personalized nutrition and therapeutic strategies targeting microbial metabolism and intestinal motility, thereby opening new avenues in both clinical and experimental gastroenterology.
Atmo Biosciences’ leadership highlights that full ownership of the technology’s core intellectual property consolidates the company’s ability to prioritize enhancements and expedite market entry timelines. The strategic equity partnership with RMIT University cements a long-term relationship that fosters ongoing innovation. Faculty and students who contributed to the original research continue to play key roles at Atmo, underscoring the importance of university-industry collaboration in driving translational science that benefits public health.
Technically, the Atmo Gas Capsule integrates multiple sensor elements capable of detecting hydrogen, methane, carbon dioxide, and other biogenic gases with high sensitivity. These sensors are interfaced with a miniaturized electronics package that controls measurement cycles and manages real-time data communication. The device’s biocompatible casing ensures safe passage through the gastrointestinal tract while protecting internal components from harsh acidic and enzymatic conditions. Advanced signal processing algorithms enable differentiation between gas profiles corresponding to various gut regions and digestive phases.
Beyond clinical diagnostics, the capsule’s rich data stream offers researchers a dynamic window into gut physiology and microbiome function. This capability facilitates detailed exploration of gut bacteria fermentation patterns, transit irregularities, and their relationship to systemic diseases such as irritable bowel syndrome and inflammatory bowel disease. Furthermore, it supports the evaluation of novel therapeutics aiming to modulate gut motility or microbial composition, enabling more targeted and effective interventions.
As Atmo advances regulatory submissions and prepares for commercialization, the company envisions a future where the Atmo Gas Capsule becomes an integral component of precision gastroenterology. By transforming gut health from a black box into a transparent, data-driven field, this technology empowers clinicians to make earlier, more accurate diagnoses, tailor treatments, and monitor patient responses over time. The implications extend beyond individual patient care to broader public health by facilitating population-level understanding of gut-related diseases.
In summary, the Atmo Gas Capsule represents a paradigm shift in gut health monitoring. Its innovative ingestion-based sensing approach harnesses real-time biomarker detection to illuminate the hidden biochemical and physiological processes within the human digestive system. Through solid academic and industry collaboration, extensive clinical validation, and cutting-edge engineering, this device is poised to redefine gastrointestinal diagnostics and research globally.
Subject of Research: Ingestible gas-sensing technology for real-time gastrointestinal biomarker monitoring and diagnosis of gut motility disorders.
Article Title: Innovative Atmo Gas Capsule Nears Market Readiness for Revolutionary Gut Health Diagnostics
News Publication Date: Information not provided
Web References: https://earimediaprodweb.azurewebsites.net/Api/v1/Multimedia/fae380c0-9ae6-464d-b72c-3405b670abc3/Rendition/low-res/Content/Public
Image Credits: Atmo
Keywords: Diseases and disorders; Technology transfer; Clinical research; Gastrointestinal disorders; Research and development; Microbiota; Human gut microbiota